Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults
- PMID: 26268313
- PMCID: PMC4669850
- DOI: 10.1126/scitranslmed.aac5745
Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults
Abstract
Respiratory syncytial virus (RSV) causes respiratory infection in annual epidemics, with infants and the elderly at particular risk of developing severe disease and death. However, despite its importance, no vaccine exists. The chimpanzee adenovirus, PanAd3-RSV, and modified vaccinia virus Ankara, MVA-RSV, are replication-defective viral vectors encoding the RSV fusion (F), nucleocapsid (N), and matrix (M2-1) proteins for the induction of humoral and cellular responses. We performed an open-label, dose escalation, phase 1 clinical trial in 42 healthy adults in which four different combinations of prime/boost vaccinations were investigated for safety and immunogenicity, including both intramuscular (IM) and intranasal (IN) administration of the adenovirus-vectored vaccine. The vaccines were safe and well tolerated, with the most common reported adverse events being mild injection site reactions. No vaccine-related serious adverse events occurred. RSV neutralizing antibody titers rose in response to IM prime with PanAd3-RSV and after IM boost for individuals primed by the IN route. Circulating anti-F immunoglobulin G (IgG) and IgA antibody-secreting cells (ASCs) were observed after the IM prime and IM boost. RSV-specific T cell responses were increased after the IM PanAd3-RSV prime and were most efficiently boosted by IM MVA-RSV. Interferon-γ (IFN-γ) secretion after boost was from both CD4(+) and CD8(+) T cells, without detectable T helper cell 2 (TH2) cytokines that have been previously associated with immune pathogenesis following exposure to RSV after the formalin-inactivated RSV vaccine. In conclusion, PanAd3-RSV and MVA-RSV are safe and immunogenic in healthy adults. These vaccine candidates warrant further clinical evaluation of efficacy to assess their potential to reduce the burden of RSV disease.
Trial registration: ClinicalTrials.gov NCT01805921.
Copyright © 2015, American Association for the Advancement of Science.
Figures

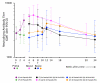
 Group 1,
Group 1, Group 2,
Group 2,  Group 3,
Group 3,  Group 4). Responses after prime (P) are grouped by the route of PanAd3-RSV administration (
Group 4). Responses after prime (P) are grouped by the route of PanAd3-RSV administration ( IM or
IM or  IN). At week 4 the IM PanAd3-RSV boost (B) was administered to group 2
IN). At week 4 the IM PanAd3-RSV boost (B) was administered to group 2 . At week 8, IM MVA-RSV and IM PanAd3-RSV boost (B) vaccines were given to the remaining volunteers. The results of individual volunteers are presented in the supplementary material (sFigure 3). The antibody titre 4 weeks following IM PanAd3-RSV prime was significantly elevated from baseline (p < 0.001, paired t-test) but not following the IN route (p = 0.816, paired t-test). For volunteers who received IM prime, the titres 4 weeks after boost were not statistically significant from pre-boost titres (group 1 between week 8 and week 12, p = 0.152; group 2 between week 4 and 8, p = 0.872; paired t-tests). Final measures of serum neutralising antibody titres were statistically indistinguishable from baseline in all groups (group 1 p=0.316, group 2 p=0.416, group 3 p=0.587, group 4 p=0.152; paired t-tests).
. At week 8, IM MVA-RSV and IM PanAd3-RSV boost (B) vaccines were given to the remaining volunteers. The results of individual volunteers are presented in the supplementary material (sFigure 3). The antibody titre 4 weeks following IM PanAd3-RSV prime was significantly elevated from baseline (p < 0.001, paired t-test) but not following the IN route (p = 0.816, paired t-test). For volunteers who received IM prime, the titres 4 weeks after boost were not statistically significant from pre-boost titres (group 1 between week 8 and week 12, p = 0.152; group 2 between week 4 and 8, p = 0.872; paired t-tests). Final measures of serum neutralising antibody titres were statistically indistinguishable from baseline in all groups (group 1 p=0.316, group 2 p=0.416, group 3 p=0.587, group 4 p=0.152; paired t-tests).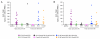
 Group 1,
Group 1, Group 2,
Group 2,  Group 3,
Group 3,  Group 4. Combined groups; by route of PanAd3-RSV prime administration
Group 4. Combined groups; by route of PanAd3-RSV prime administration  IM and
IM and  IN.
IN.
 Group 1,
Group 1, Group 2,
Group 2,  Group 3,
Group 3,  Group 4. Combined groups; by route of PanAd3-RSV prime administration
Group 4. Combined groups; by route of PanAd3-RSV prime administration  IM and
IM and  IN.
IN.
 Group 1,
Group 1, Group 2,
Group 2,  Group 3,
Group 3,  Group 4. The frequency of responses is presented in supplementary material (sFigure 8).
Group 4. The frequency of responses is presented in supplementary material (sFigure 8).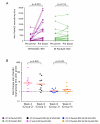
 Group 1,
Group 1, Group 2,
Group 2,  Group 3,
Group 3,  Group 4. Combined groups; by route of PanAd3-RSV prime administration
Group 4. Combined groups; by route of PanAd3-RSV prime administration  IM and
IM and  IN.
IN.Comment in
-
Early promise for infant RSV vaccine development.Lancet Respir Med. 2015 Sep;3(9):676. doi: 10.1016/S2213-2600(15)00328-8. Epub 2015 Aug 23. Lancet Respir Med. 2015. PMID: 26310688 No abstract available.
-
Virus vs. virus: adenovirus vectored vaccine to defeat respiratory syncytial virus.Ann Transl Med. 2016 Dec;4(24):489. doi: 10.21037/atm.2016.12.08. Ann Transl Med. 2016. PMID: 28149851 Free PMC article. No abstract available.
References
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. American journal of diseases of children. 1986;140:543–546. - PubMed
-
- Miller EK, Gebretsadik T, Carroll KN, Dupont WD, Mohamed YA, Morin LL, Heil L, Minton PA, Woodward K, Liu Z, Hartert TV, Williams JV. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. The Pediatric infectious disease journal. 2013;32:950–955. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

