Restrictive IgG antibody response against mutated citrullinated vimentin predicts response to rituximab in patients with rheumatoid arthritis
- PMID: 26268352
- PMCID: PMC4535682
- DOI: 10.1186/s13075-015-0717-z
Restrictive IgG antibody response against mutated citrullinated vimentin predicts response to rituximab in patients with rheumatoid arthritis
Abstract
Introduction: Antibodies against mutated citrullinated vimentin (AMCV) represent a useful diagnostic marker with correlation to disease activity in patients with rheumatoid arthritis (RA). Since seropositivity for citrullinated autoantibodies was predictive for response to B-cell depleting therapy (BCDT) with rituximab (RTX), we investigated whether differences in antibody fine reactivity and immunoglobulin (Ig) isotype kinetics among AMCV-positive patients could provide additional information about outcome.
Methods: A total of 50 AMCV IgG-positive RA patients (RTX responders (RRs) n = 37 and non-responders (NRRs) n = 13) were analyzed for reactivity against MCV epitopes and co-existent AMCV isotypes IgM and IgA. Antibody titers were determined by enzyme-linked immunosorbent assay at baseline and 24 weeks after the first cycle of RTX, and compared to kinetics of rheumatoid factor (RF) and antibodies against cyclic citrullinated peptide (ACCP).
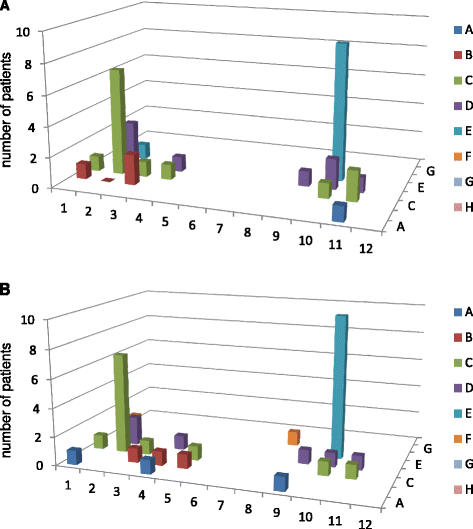
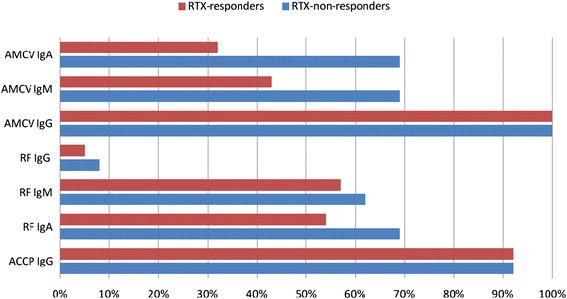
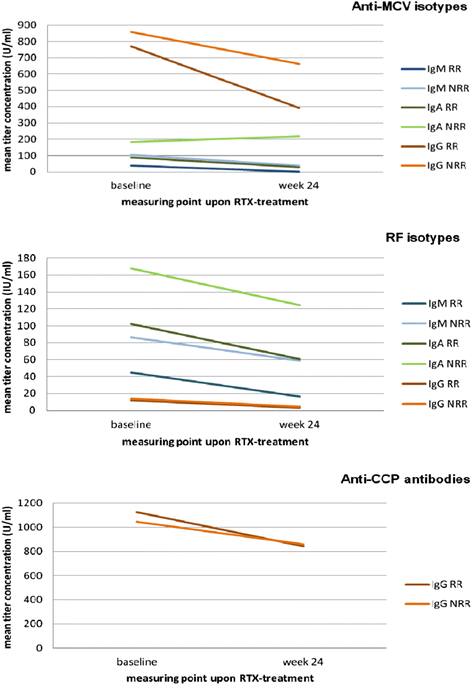
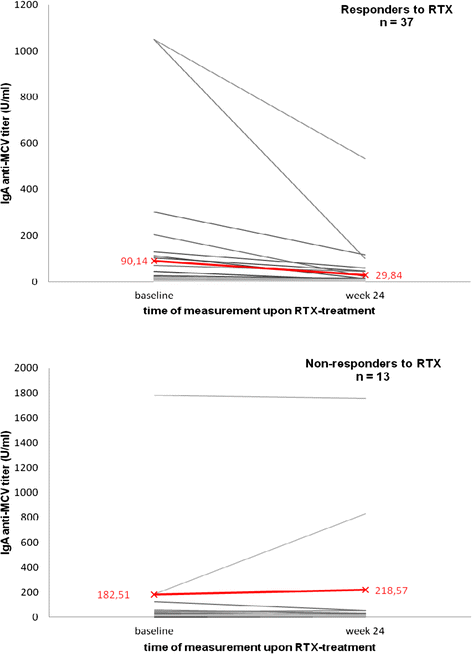
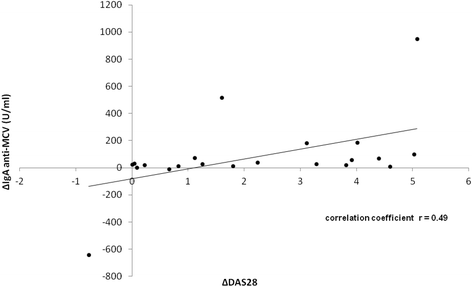
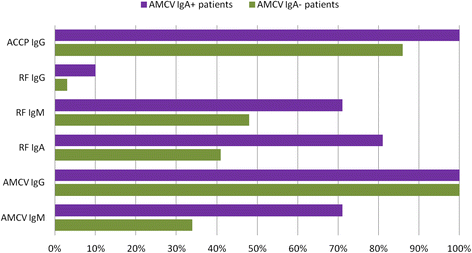
Results: Recognized MCV epitopes by AMCV IgG of RRs and NRRs showed similar baseline patterns, with reducing reactivity in RRs and unchanged or even expanding reactivity in NRRs upon RTX treatment. At baseline, RRs were more frequently negative for AMCV subtypes, especially for IgA (68%), compared to NRRs (31%). Being AMCV IgA-negative at baseline indicated a good treatment response to RTX (negative predictive value = 0.86). Co-existence of AMCV IgA and IgG with stable titers upon treatment were associated with poorer responses to RTX. Furthermore, reductions of AMCV IgA levels upon RTX correlated with the improvement of 28-joint Disease Activity Score (DAS28). In comparison, subtypes of RF and ACCP were not of additional value for prediction of RTX response.
Conclusions: Restrictive IgG seropositivity against MCV with treatment-associated decline in fine reactivity and titers was predictive for response to RTX. Double-positivity for AMCV IgG and IgA was associated with failure to respond to BCDT, suggesting a pathogenetic and less sensitive IgA-producing B-cell subset in NRRs.
Figures


References
-
- Bykerk VP, Keystone EC, Kuriya B, Larche M, Thorne JC, Haraoui B. Achieving remission in clinical practice: lessons from clinical trial data. Clin Exp Rheumatol. 2013;31:621–32. - PubMed
-
- Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

