p75(NTR)-dependent activation of NF-κB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia
- PMID: 26268439
- PMCID: PMC4538859
- DOI: 10.1038/ncomms9024
p75(NTR)-dependent activation of NF-κB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia
Abstract
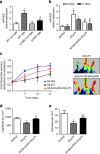
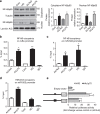
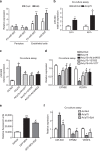
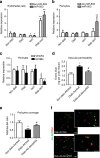
The communication between vascular endothelial cells (ECs) and pericytes in the microvasculature is fundamental for vascular growth and homeostasis; however, these processes are disrupted by diabetes. Here we show that modulation of p75(NTR) expression in ECs exposed to high glucose activates transcription of miR-503, which negatively affects pericyte function. p75(NTR) activates NF-κB to bind the miR-503 promoter and upregulate miR-503 expression in ECs. NF-κB further induces activation of Rho kinase and shedding of endothelial microparticles carrying miR-503, which transfer miR-503 from ECs to vascular pericytes. The integrin-mediated uptake of miR-503 in the recipient pericytes reduces expression of EFNB2 and VEGFA, resulting in impaired migration and proliferation. We confirm operation of the above mechanisms in mouse models of diabetes, in which EC-derived miR-503 reduces pericyte coverage of capillaries, increased permeability and impaired post-ischaemic angiogenesis in limb muscles. Collectively, our data demonstrate that miR-503 regulates pericyte-endothelial crosstalk in microvascular diabetic complications.
Figures





References
-
- Dinh T., Scovell S. & Veves A. Peripheral arterial disease and diabetes: a clinical update. Int. J. Low. Extrem. Wounds 8, 75–81 (2009). - PubMed
-
- Hinchliffe R. J. et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes Metab. Res. Rev. 28, (Suppl 1): 179–217 (2012). - PubMed
-
- Emanueli C. et al. Prevention of diabetes-induced microangiopathy by human tissue kallikrein gene transfer. Circulation 106, 993–999 (2002). - PubMed
-
- Hammes H. P. et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51, 3107–3112 (2002). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

