Assessing the effectiveness of household-level focal mass drug administration and community-wide mass drug administration for reducing malaria parasite infection prevalence and incidence in Southern Province, Zambia: study protocol for a community randomized controlled trial
- PMID: 26268804
- PMCID: PMC4535296
- DOI: 10.1186/s13063-015-0862-3
Assessing the effectiveness of household-level focal mass drug administration and community-wide mass drug administration for reducing malaria parasite infection prevalence and incidence in Southern Province, Zambia: study protocol for a community randomized controlled trial
Abstract
Background: Mass drug administration (MDA) and focal MDA (fMDA) using dihydroartemisinin plus piperaquine (DHAp), represent two strategies to maximize the use of existing information to achieve greater clearance of human infection and reduce the parasite reservoir, and provide longer chemoprophylactic protection against new infections. The primary aim of this study is to quantify the relative effectiveness of MDA and fMDA with DHAp against no mass treatment (standard of care) for reducing Plasmodium falciparum prevalence and incidence.
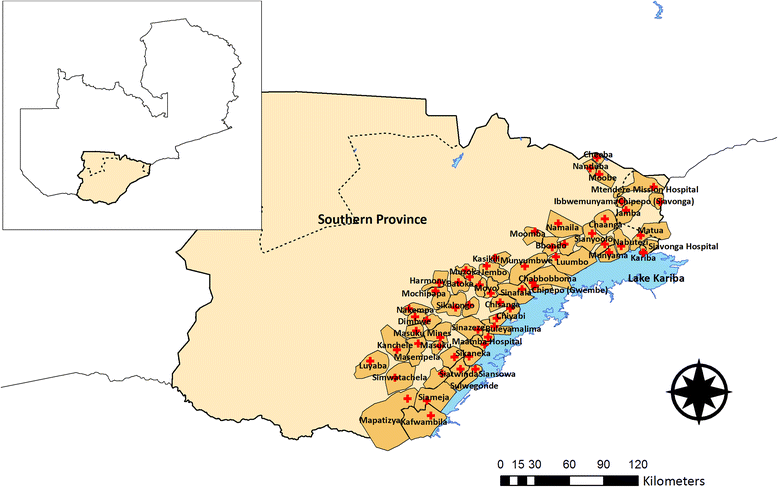
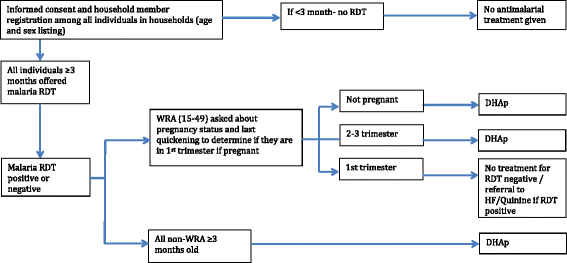
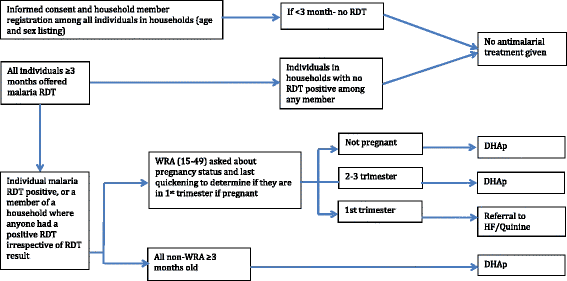
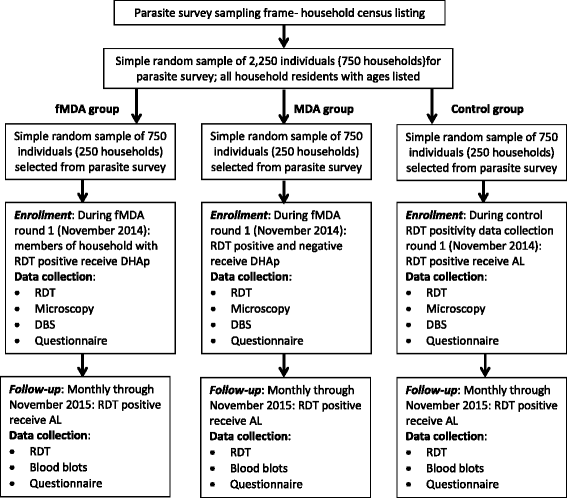
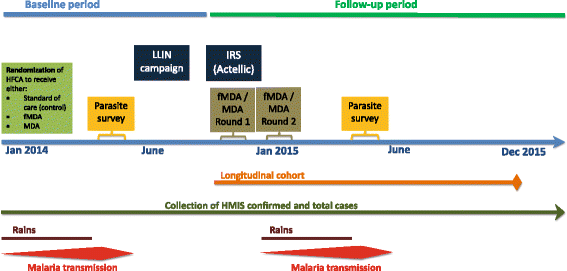
Methods/design: The study will be conducted along Lake Kariba in Southern Province, Zambia; an area of low to moderate malaria transmission and high coverage of vector control. A community randomized controlled trial (CRCT) of 60 health facility catchment areas (HFCAs) will be used to evaluate the impact of two rounds of MDA and fMDA interventions, relative to a control of no mass treatment, stratified by high and low transmission. Community residents in MDA HFCAs will be treated with DHAp at the end of the dry season (round one: November to December 2014) and the beginning of the rainy season (round two: February to March 2015). Community residents in fMDA HFCAs will be tested during the same two rounds for malaria parasites with a rapid diagnostic test; all positive individuals and all individuals living in their household will be treated with DHAp. Primary outcomes include malaria parasite prevalence (n = 5,640 children aged one month to under five-years-old), as measured by pre- and post-surveys, and malaria parasite infection incidence (n = 2,250 person-years among individuals aged three months and older), as measured by a monthly longitudinal cohort. The study is powered to detect approximately a 50 % relative reduction in these outcomes between each intervention group versus the control.
Discussion: Strengths of this trial include: a robust study design (CRCT); cross-sectional parasite surveys as well as a longitudinal cohort; and stratification of high and low transmission areas. Primary limitations include: statistical power to detect only a 50 % reduction in primary outcomes within high and low transmission strata; potential for contamination; and potential for misclassification of exposure.
Trial registration: Identifier: Clinicaltrials.gov: NCT02329301 . Registration date: 30 December 2014.
Figures
References
-
- Zambia Ministry of Health. National Malaria Control Programme Strategic Plan for FY 2011–2015: Consolidating malaria gains for impact. Lusaka: Zambia Ministry of Public Health; 2011
-
- Larsen DA, Bennett A, Silumbe K, Hamainza B, Yukich JO, Keating J, et al. Population-wide malaria testing and treatment with rapid diagnostic tests and artemether-lumefantrine in southern zambia: a community randomized step-wedge control trial design. Am J Trop Med Hyg. 2015;92(5):913–21. doi: 10.4269/ajtmh.14-0347. - DOI - PMC - PubMed
-
- World Health Organization (WHO) Guidelines for the treatment of malaria. 2. Geneva: WHO; 2010. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

