Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: an observational study
- PMID: 26268818
- PMCID: PMC4535562
- DOI: 10.1186/s12885-015-1525-1
Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: an observational study
Abstract
Background: The aim of this study was to evaluate the survival benefit of radical surgery with additional extensive upper abdominal procedures (EUAS) for the treatment of stage IIIC and IV ovarian cancer with bulky upper abdominal disease (UAD).
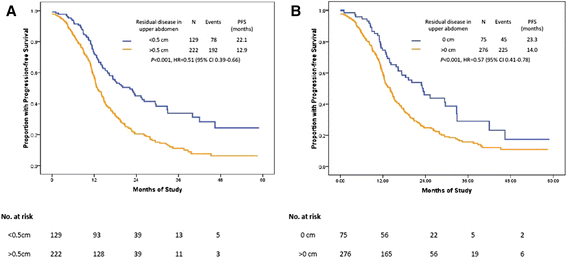
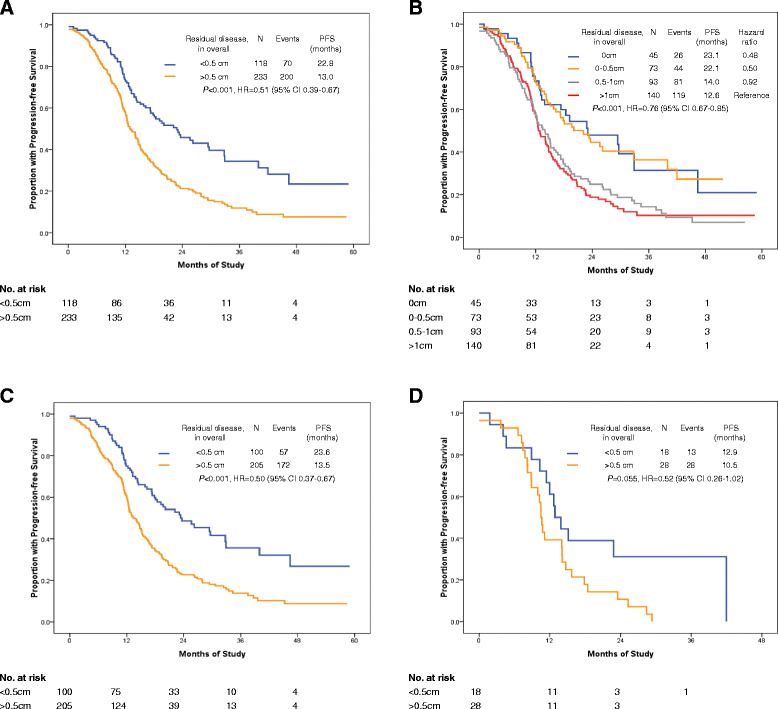
Methods: An observational study was conducted between 2009 and 2012 involving two different surgical teams. Team A was composed of the "believers" in EUAS and Team B the "non-believers" in EUAS. Patients were divided into a radical surgery group (EUAS group) or a standard surgery group (non-EUAS group) according to whether or not they had received EUAS. All patients underwent primary cytoreductive surgery with the goal of optimal debulking (≤ 1 cm); this was reviewed in the pelvis, middle abdomen, and upper abdomen. The baseline for the two groups was optimal cytoreduction in both the pelvis and middle abdomen. Progression-free survival (PFS) was evaluated.
Results: Radical surgery was performed in 70.7% (82/116) and 12.7% (30/237) of the patients by Teams A and B, respectively. The study groups had similar clinicopathologic characteristics. The median PFS and OS were significantly improved in the radical surgery group, compared with standard surgery groups (PFS: 19.5 vs. 13.3 months, HR: 0.61; 95% CI: 0.46-0.80, P < 0.001; OS: not reached vs. 39.3 months, HR: 0.47; 95% CI: 0.30-0.72, P < 0.001). Positive predictors of complete cytoreduction were treatment with neoadjuvant chemotherapy, improved American Society of Anesthesiologists performance status, and the absence of bowel mesenteric carcinomatosis.
Conclusions: Radical surgery lengthens the PFS and overall survival times of ovarian cancer patients with bulky UAD. However, a well-designed randomized trial is needed to confirm the present results.
Figures




References
-
- Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, Sonoda Y, Levine DA, Hensley M, Barakat RR. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. - DOI - PubMed
-
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelialovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–44. doi: 10.1002/cncr.24149. - DOI - PubMed
-
- Rodriguez N, Miller A, Richard SD, Rungruang B, Hamilton CA, Bookman MA, Maxwell GL, Horowitz NS, Krivak TC. Upper abdominal procedures in advanced stage ovarian or primary peritoneal carcinoma patients with minimal or no gross residual disease: an analysis of Gynecologic Oncology Group (GOG) 182. Gynecol Oncol. 2013;130:487–92. doi: 10.1016/j.ygyno.2013.06.017. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

