Slow-growing cells within isogenic populations have increased RNA polymerase error rates and DNA damage
- PMID: 26268986
- PMCID: PMC4557116
- DOI: 10.1038/ncomms8972
Slow-growing cells within isogenic populations have increased RNA polymerase error rates and DNA damage
Abstract
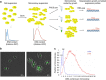
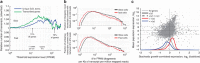
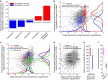
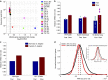
Isogenic cells show a large degree of variability in growth rate, even when cultured in the same environment. Such cell-to-cell variability in growth can alter sensitivity to antibiotics, chemotherapy and environmental stress. To characterize transcriptional differences associated with this variability, we have developed a method--FitFlow--that enables the sorting of subpopulations by growth rate. The slow-growing subpopulation shows a transcriptional stress response, but, more surprisingly, these cells have reduced RNA polymerase fidelity and exhibit a DNA damage response. As DNA damage is often caused by oxidative stress, we test the addition of an antioxidant, and find that it reduces the size of the slow-growing population. More generally, we find a significantly altered transcriptome in the slow-growing subpopulation that only partially resembles that of cells growing slowly due to environmental and culture conditions. Slow-growing cells upregulate transposons and express more chromosomal, viral and plasmid-borne transcripts, and thus explore a larger genotypic--and so phenotypic--space.
Figures
References
-
- Kiviet D. J. et al.. Stochasticity of metabolism and growth at the single-cell level. Nature 514, 376–379 (2014). - PubMed
-
- Wakamoto Y. et al.. Dynamic persistence of antibiotic-stressed mycobacteria. Science 339, 91–95 (2013). - PubMed
-
- Balaban N. Q., Gerdes K., Lewis K. & McKinney J. D. A problem of persistence: still more questions than answers? Nat. Rev. Microbiol. 11, 587–591 (2013). - PubMed
-
- Fridman O., Goldberg A., Ronin I., Shoresh N. & Balaban N. Q. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 513, 418–421 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

