Mapping molecules in scanning far-field fluorescence nanoscopy
- PMID: 26269133
- PMCID: PMC4557268
- DOI: 10.1038/ncomms8977
Mapping molecules in scanning far-field fluorescence nanoscopy
Abstract
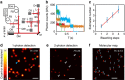
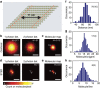
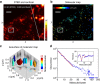
In fluorescence microscopy, the distribution of the emitting molecule number in space is usually obtained by dividing the measured fluorescence by that of a single emitter. However, the brightness of individual emitters may vary strongly in the sample or be inaccessible. Moreover, with increasing (super-) resolution, fewer molecules are found per pixel, making this approach unreliable. Here we map the distribution of molecules by exploiting the fact that a single molecule emits only a single photon at a time. Thus, by analysing the simultaneous arrival of multiple photons during confocal imaging, we can establish the number and local brightness of typically up to 20 molecules per confocal (diffraction sized) recording volume. Subsequent recording by stimulated emission depletion microscopy provides the distribution of the number of molecules with subdiffraction resolution. The method is applied to mapping the three-dimensional nanoscale organization of internalized transferrin receptors on human HEK293 cells.
Figures
References
-
- Abbe E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Archiv für Mikroskopische Anatomie 9, 413–468 (1873).
-
- Hell S. W. Far-field optical nanoscopy. Science 316, 1153–1158 (2007). - PubMed
-
- Hell S. W. & Wichmann J. Breaking the diffraction resolution limit by stimulated-emission—stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

