Viral Source-Independent High Susceptibility of Dendritic Cells to Human T-Cell Leukemia Virus Type 1 Infection Compared to That of T Lymphocytes
- PMID: 26269171
- PMCID: PMC4580172
- DOI: 10.1128/JVI.01799-15
Viral Source-Independent High Susceptibility of Dendritic Cells to Human T-Cell Leukemia Virus Type 1 Infection Compared to That of T Lymphocytes
Abstract
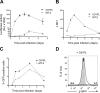
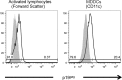
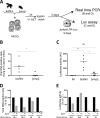
Human T-cell leukemia virus type 1 (HTLV-1)-infected CD4(+) T cells and dendritic cells (DCs) are present in peripheral blood from HTLV-1 carriers. While T-cell infection requires cell-cell contact, DCs might be infected with cell-free virus, at least in vitro. However, a thorough comparison of the susceptibilities of the two cell types to HTLV-1 infection using cell-associated and cell-free viral sources has not been performed. We first determined that human primary monocyte-derived dendritic cells (MDDCs) were more susceptible to HTLV-1 infection than their autologous lymphocyte counterparts after contact with chronically infected cells. Next, a comparison of infection efficiency using nonconcentrated or concentrated supernatants from infected cells as well as purified viral biofilm was performed. Integrated provirus was found after exposure of MDDCs or primary lymphocytes to viral biofilm but not to a viral supernatant. Using a large series of primary cell samples (n = 21), we demonstrated a higher proviral load in MDDCs exposed to viral biofilm than in lymphocytes. This higher susceptibility is correlated to a higher expression of neuropilin-1 on MDDCs than on autologous activated T lymphocytes. Moreover, we show that MDDCs infected with viral biofilm can transmit the virus to lymphocytes. In conclusion, MDDCs are more susceptible to HTLV-1 infection than autologous lymphocytes in vitro, supporting a model in which DC infection might represent an important step during primo-infection in vivo.
Importance: HTLV-1 is able to infect several cell types, but viral DNA is mainly found in T lymphocytes in vivo. This supports a model in which T lymphocytes are the main target of infection. However, during the primo-infection of new individuals, incoming viruses might first encounter dendritic cells (DCs), the specialized immune cells responsible for the antiviral response of the host. HTLV-1 cell-free purified viruses can infect dendritic cells in vitro, while T-cell infection is restricted to cell-to-cell transmission. In order to understand the sequence of HTLV-1 dissemination, we undertook a direct comparison of the susceptibilities of the two cell types using cell-associated and cell-free viral sources. We report here that MDDCs are more susceptible to HTLV-1 infection than autologous lymphocytes in vitro and are able to efficiently transmit the virus to lymphocytes. Our results suggest that DCs may represent a true viral reservoir, as the first cell type to be infected in vivo.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Gessain A, Barin F, Vernant J, Gout O, Maurs L, Calender A, de Thé G. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407–410. - PubMed
-
- Osame M, Igata A, Matsumoto M, Izumo S, Usuku K, Rosales RL, Tara M. 1988. HTLV-1 associated myelopathy (HAM). Kansenshogaku Zasshi 62(Suppl):240–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

