MicroRNA-Attenuated Clone of Virulent Semliki Forest Virus Overcomes Antiviral Type I Interferon in Resistant Mouse CT-2A Glioma
- PMID: 26269187
- PMCID: PMC4580204
- DOI: 10.1128/JVI.01868-15
MicroRNA-Attenuated Clone of Virulent Semliki Forest Virus Overcomes Antiviral Type I Interferon in Resistant Mouse CT-2A Glioma
Abstract
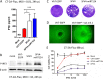
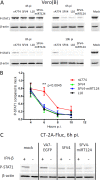
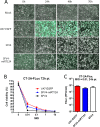
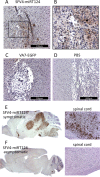
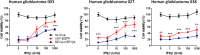
Glioblastoma is a terminal disease with no effective treatment currently available. Among the new therapy candidates are oncolytic viruses capable of selectively replicating in cancer cells, causing tumor lysis and inducing adaptive immune responses against the tumor. However, tumor antiviral responses, primarily mediated by type I interferon (IFN-I), remain a key problem that severely restricts viral replication and oncolysis. We show here that the Semliki Forest virus (SFV) strain SFV4, which causes lethal encephalitis in mice, is able to infect and replicate independent of the IFN-I defense in mouse glioblastoma cells and cell lines originating from primary human glioblastoma patient samples. The ability to tolerate IFN-I was retained in SFV4-miRT124 cells, a derivative cell line of strain SFV4 with a restricted capacity to replicate in neurons due to insertion of target sites for neuronal microRNA 124. The IFN-I tolerance was associated with the viral nsp3-nsp4 gene region and distinct from the genetic loci responsible for SFV neurovirulence. In contrast to the naturally attenuated strain SFV A7(74) and its derivatives, SFV4-miRT124 displayed increased oncolytic potency in CT-2A murine astrocytoma cells and in the human glioblastoma cell lines pretreated with IFN-I. Following a single intraperitoneal injection of SFV4-miRT124 into C57BL/6 mice bearing CT-2A orthotopic gliomas, the virus homed to the brain and was amplified in the tumor, resulting in significant tumor growth inhibition and improved survival.
Importance: Although progress has been made in development of replicative oncolytic viruses, information regarding their overall therapeutic potency in a clinical setting is still lacking. This could be at least partially dependent on the IFN-I sensitivity of the viruses used. Here, we show that the conditionally replicating SFV4-miRT124 virus shares the IFN-I tolerance of the pathogenic wild-type SFV, thereby allowing efficient targeting of a glioma that is refractory to naturally attenuated therapy vector strains sensitive to IFN-I. This is the first evidence of orthotopic syngeneic mouse glioma eradication following peripheral alphavirus administration. Our findings indicate a clear benefit in harnessing the wild-type virus replicative potency in development of next-generation oncolytic alphaviruses.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. 2005. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330. - DOI - PubMed
-
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, Nabors LB, Markiewicz M, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, Gillespie GY. 2009. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre- and post-tumor resection for recurrent GBM. Mol Ther 17:199–207. doi:10.1038/mt.2008.228. - DOI - PMC - PubMed
-
- Freeman A, Zakay-Rones Z, GomoriI J, Linetsky E, Rasooly L, Greenbaum E, Rozenman-Yair S, Panet A, Libson E, Irving C, Galun E, Siegal T. 2006. Phase I/II trial of Intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther 13:221–228. doi:10.1016/j.ymthe.2005.08.016. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

