The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells
- PMID: 26269243
- PMCID: PMC4580964
- DOI: 10.3945/jn.115.218586
The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells
Abstract
Background: MicroRNAs play essential roles in gene regulation. A substantial fraction of microRNAs in tissues and body fluids is encapsulated in exosomes, thereby conferring protection against degradation and a pathway for intestinal transport. MicroRNAs in cow milk are bioavailable in humans.
Objective: This research assessed the transport mechanism of bovine milk exosomes, and therefore microRNAs, in human and rodent intestinal cells.
Methods: The intestinal transport of bovine milk exosomes and microRNAs was assessed using fluorophore-labeled bovine milk exosomes in human colon carcinoma Caco-2 cells and rat small intestinal IEC-6 cells. Transport kinetics and mechanisms were characterized using dose-response studies, inhibitors of vesicle transport, carbohydrate competitors, proteolysis of surface proteins on cells and exosomes, and transepithelial transport in transwell plates.
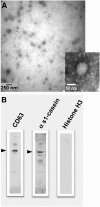
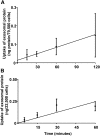
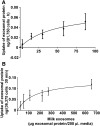
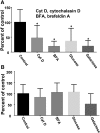
Results: Exosome transport exhibited saturation kinetics at 37°C [Michaelis constant (Km) = 55.5 ± 48.6 μg exosomal protein/200 μL of media; maximal transport rate = 0.083 ± 0.057 ng of exosomal protein · 81,750 cells(-1) · h(-1)] and decreased by 64% when transport was measured at 4°C, consistent with carrier-mediated transport in Caco-2 cells. Exosome uptake decreased by 61-85% under the following conditions compared with controls in Caco-2 cells: removal of exosome and cell surface proteins by proteinase K, inhibition of endocytosis and vesicle trafficking by synthetic inhibitors, and inhibition of glycoprotein binding by carbohydrate competitors. When milk exosomes, at a concentration of 5 times the Km, were added to the upper chamber in transwell plates, Caco-2 cells accumulated miR-29b and miR-200c in the lower chamber, and reverse transport was minor. Transport characteristics were similar in IEC-6 cells and Caco-2 cells, except that substrate affinity and transporter capacity were lower and higher, respectively.
Conclusion: The uptake of bovine milk exosomes is mediated by endocytosis and depends on cell and exosome surface glycoproteins in human and rat intestinal cells.
Keywords: endocytosis; extracellular vesicles; intestinal cells; milk exosomes; uptake.
© 2015 American Society for Nutrition.
Conflict of interest statement
Author disclosures: T Wolf, SR Baier, and J Zempleni, no conflicts of interest. The granting agencies had no influence on the study design; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Figures
References
-
- Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 2007;8:93–103. - PubMed
-
- Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 2005;120:623–34. - PubMed
-
- Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol 2007;8:23–36. - PubMed
-
- Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol 2013;14:475–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

