The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology
- PMID: 26269524
- PMCID: PMC4600949
- DOI: 10.1152/physrev.00001.2015
The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology
Abstract
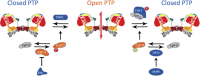
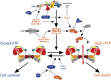
The mitochondrial permeability transition (PT) is a permeability increase of the inner mitochondrial membrane mediated by a channel, the permeability transition pore (PTP). After a brief historical introduction, we cover the key regulatory features of the PTP and provide a critical assessment of putative protein components that have been tested by genetic analysis. The discovery that under conditions of oxidative stress the F-ATP synthases of mammals, yeast, and Drosophila can be turned into Ca(2+)-dependent channels, whose electrophysiological properties match those of the corresponding PTPs, opens new perspectives to the field. We discuss structural and functional features of F-ATP synthases that may provide clues to its transition from an energy-conserving into an energy-dissipating device as well as recent advances on signal transduction to the PTP and on its role in cellular pathophysiology.
Copyright © 2015 the American Physiological Society.
Figures


References
-
- Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621–628, 1994. - PubMed
-
- Acin-Pérez R, Carrascoso I, Baixauli F, Roche-Molina M, Latorre-Pellicer A, Fernández-Silva P, Mittelbrunn M, Sanchez-Madrid F, Pérez-Martos A, Lowell CA, Manfredi G, Enríquez JA. ROS-triggered phosphorylation of complex II by Fgr kinase regulates cellular adaptation to fuel use. Cell Metab 19: 1020–1033, 2014. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

