Neutrophil elastase promotes interleukin-1β secretion from human coronary endothelium
- PMID: 26269588
- PMCID: PMC4591798
- DOI: 10.1074/jbc.M115.659029
Neutrophil elastase promotes interleukin-1β secretion from human coronary endothelium
Abstract
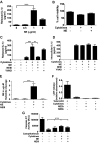
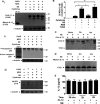
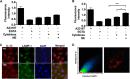
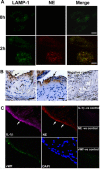
The endothelium is critically involved in the pathogenesis of atherosclerosis by producing pro-inflammatory mediators, including IL-1β. Coronary arteries from patients with ischemic heart disease express large amounts of IL-1β in the endothelium. However, the mechanism by which endothelial cells (ECs) release IL-1β remains to be elucidated. We investigated neutrophil elastase (NE), a potent serine protease detected in vulnerable areas of human carotid plaques, as a potential "trigger" for IL-1β processing and release. This study tested the hypothesis that NE potentiates the processing and release of IL-1β from human coronary endothelium. We found that NE cleaves the pro-isoform of IL-1β in ECs and causes significant secretion of bioactive IL-1β via extracellular vesicles. This release was attenuated significantly by inhibition of neutrophil elastase but not caspase-1. Transient increases in intracellular Ca(2+) levels were observed prior to secretion. Inside ECs, and after NE treatment only, IL-1β was detected within LAMP-1-positive multivesicular bodies. The released vesicles contained bioactive IL-1β. In vivo, in experimental atherosclerosis, NE was detected in mature atherosclerotic plaques, predominantly in the endothelium, alongside IL-1β. This study reveals a novel mechanistic link between NE expression in atherosclerotic plaques and concomitant pro-inflammatory bioactive IL-1β secretion from ECs. This could reveal additional potential anti-IL-1β therapeutic targets and provide further insights into the inflammatory process by which vascular disease develops.
Keywords: IL-1; atherosclerosis; endothelium; extracellular vesicles; inflammation; neutrophil elastase.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Libby P. (2002) Inflammation in atherosclerosis. Nature 420, 868–874 - PubMed
-
- Bonetti P. O., Lerman L. O., Lerman A. (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 23, 168–175 - PubMed
-
- Ridker P. M., Thuren T., Zalewski A., Libby P. (2011) Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 162, 597–605 - PubMed
-
- Dinarello C. A. (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

