Biodistribution and pharmacokinetics of recombinant α1-microglobulin and its potential use in radioprotection of kidneys
- PMID: 26269772
- PMCID: PMC4529588
Biodistribution and pharmacokinetics of recombinant α1-microglobulin and its potential use in radioprotection of kidneys
Abstract
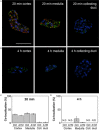
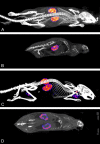
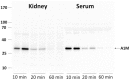
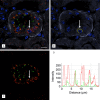
Peptide-receptor radionuclide therapy (PRRT) is a systemically administrated molecular targeted radiation therapy for treatment of neuroendocrine tumors. Fifteen years of clinical use show that renal toxicity, due to glomerular filtration of the peptides followed by local generation of highly reactive free radicals, is the main side-effect that limits the maximum activity that can be administrated for efficient therapy. α1-microglobulin (A1M) is an endogenous radical scavenger shown to prevent radiation-induced in vitro cell damage and protect non-irradiated surrounding cells. An important feature of A1M is that, following distribution to the blood, it is equilibrated to the extravascular compartments and filtrated in the kidneys. Aiming at developing renal protection against toxic side-effects of PRRT, we have characterized the pharmacokinetics and biodistribution of intravenously (i.v.) injected (125)I- and non-labelled recombinant human A1M and the (111)In- and fluorescence-labelled somatostatin analogue octreotide. Both molecules were predominantly localized to the kidneys, displaying a prevailing distribution in the cortex. A maximum of 76% of the injected A1M and 46% of the injected octreotide were present per gram kidney tissue at 10 to 20 minutes, respectively, after i.v. injection. Immunohistochemistry and fluorescence microscopy revealed a dominating co-existence of the two substances in proximal tubules, with a cellular co-localization in the epithelial cells. Importantly, analysis of kidney extracts displayed an intact, full-length A1M at least up to 60 minutes post-injection (p.i.). In summary, the results show a highly similar pharmacokinetics and biodistribution of A1M and octreotide, thus enabling the use of A1M to protect the kidneys tissue during PRRT.
Keywords: A1M; antioxidation; glomerulus; kidney; octreotide; oxidative stress; prrt; radioprotection; tubule.
Figures





References
-
- Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Horsch D, O’Dorisio MS, O’Dorisio TM, Howe JR, Cremonesi M, Kwekkeboom DJ, Zaknun JJ. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. - PMC - PubMed
-
- Krenning EP, Bakker WH, Breeman WA, Koper JW, Kooij PP, Ausema L, Lameris JS, Reubi JC, Lamberts SW. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;1:242–244. - PubMed
-
- Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, Baio SM, Sansovini M, Paganelli G. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–1856. - PubMed
-
- de Jong M, Krenning E. New advances in peptide receptor radionuclide therapy. J Nucl Med. 2002;43:617–620. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
