Lipid Binding of the Amphipathic Helix Serving as Membrane Anchor of Pestivirus Glycoprotein Erns
- PMID: 26270479
- PMCID: PMC4536213
- DOI: 10.1371/journal.pone.0135680
Lipid Binding of the Amphipathic Helix Serving as Membrane Anchor of Pestivirus Glycoprotein Erns
Abstract
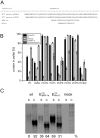
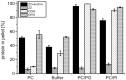
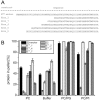
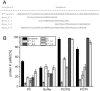
Pestiviruses express a peculiar protein named Erns representing envelope glycoprotein and RNase, which is important for control of the innate immune response and persistent infection. The latter functions are connected with secretion of a certain amount of Erns from the infected cell. Retention/secretion of Erns is most likely controlled by its unusual membrane anchor, a long amphipathic helix attached in plane to the membrane. Here we present results of experiments conducted with a lipid vesicle sedimentation assay able to separate lipid-bound from unbound protein dissolved in the water phase. Using this technique we show that a protein composed of tag sequences and the carboxyterminal 65 residues of Erns binds specifically to membrane vesicles with a clear preference for compositions containing negatively charged lipids. Mutations disturbing the helical folding and/or amphipathic character of the anchor as well as diverse truncations and exchange of amino acids important for intracellular retention of Erns had no or only small effects on the proteins membrane binding. This result contrasts the dramatically increased secretion rates observed for Erns proteins with equivalent mutations within cells. Accordingly, the ratio of secreted versus cell retained Erns is not determined by the lipid affinity of the membrane anchor.
Conflict of interest statement
Figures




References
-
- Thiel HJ, Plagemann PGW, Moennig V. Pestiviruses In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 3rd Philadelphia, New York: Lippincott—Raven Publishers; 1996. p. 1059–73.
-
- Simmonds P, Becher P, Collett MS, Gould EA, Heinz FX, Meyers G, et al. Flaviviridae In: King AMQ, Lefkowitz E, Adams MJ, Carstens EB, Fauquet CM, editors. Virus Taxonomy Ninth Report of the International Committee on Taxonomy of Viruses. San Diego, USA: Academic Press; 2012. p. 1003–20.
-
- Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: The Viruses and Their Replication In: Knipe DM, Howley PM, editors. Fields Virology. 5th Philadelphia, New York: Lippincott—Raven Publishers; 2007. p. 1101–52.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

