Synthesis of Peptides Containing C-Terminal Esters Using Trityl Side-Chain Anchoring: Applications to the Synthesis of C-Terminal Ester Analogs of the Saccharomyces cerevisiae Mating Pheromone a-Factor
- PMID: 26270598
- PMCID: PMC5035043
- DOI: 10.1021/acs.joc.5b01376
Synthesis of Peptides Containing C-Terminal Esters Using Trityl Side-Chain Anchoring: Applications to the Synthesis of C-Terminal Ester Analogs of the Saccharomyces cerevisiae Mating Pheromone a-Factor
Abstract
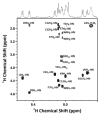
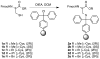
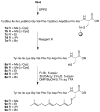
Peptides containing C-terminal esters are an important class of bioactive molecules that includes a-factor, a farnesylated dodecapeptide, involved in the mating of Saccharomyces cerevisiae. Here, results that expand the scope of solid-phase peptide synthetic methodology that uses trityl side-chain anchoring for the preparation of peptides with C-terminal cysteine alkyl esters are described. In this method, Fmoc-protected C-terminal cysteine esters are anchored to trityl chloride resin and extended by standard solid-phase procedures followed by acidolytic cleavage and HPLC purification. Analysis using a Gly-Phe-Cys-OMe model tripeptide revealed minimal epimerization of the C-terminal cysteine residue under basic conditions used for Fmoc deprotection. (1)H NMR analysis of the unfarnesylated a-factor precursor peptide confirmed the absence of epimerization. The side-chain anchoring method was used to produce wild-type a-factor that contains a C-terminal methyl ester along with ethyl-, isopropyl-, and benzyl-ester analogs in good yield. Activity assays using a yeast-mating assay demonstrate that while the ethyl and isopropyl esters manifest near-wild-type activity, the benzyl ester-containing analog is ca. 100-fold less active. This simple method opens the door to the synthesis of a variety of C-terminal ester-modified peptides that should be useful in studies of protein prenylation and other structurally related biological processes.
Figures




References
-
- Kaspar AA, Reichert JM. Drug Discovery Today. 2013;18:807. - PubMed
-
- Grauer A, Koenig B. Eur J Org Chem. 2009:5099.
-
- Gongora-Benitez M, Tulla-Puche J, Albericio F. ACS Comb Sci. 2013;15:217. - PubMed
-
- Zablocki JA, Tjoeng FS, Bovy PR, Miyano M, Garland RB, Williams K, Schretzman L, Zupec ME, Rico JG, Lindmark RJ, Toth MV, Mcmackins DE, Adams SP, Panzerknodle SG, Nicholson NS, Taite BB, Salyers AK, King LW, Campion JG, Feigen LP. Biorg Med Chem. 1995;3:539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

