Should HFE p.C282Y homozygotes with moderately elevated serum ferritin be treated? A randomised controlled trial comparing iron reduction with sham treatment (Mi-iron)
- PMID: 26270952
- PMCID: PMC4538285
- DOI: 10.1136/bmjopen-2015-008938
Should HFE p.C282Y homozygotes with moderately elevated serum ferritin be treated? A randomised controlled trial comparing iron reduction with sham treatment (Mi-iron)
Abstract
Introduction: HFE p.C282Y homozygosity is the most common cause of hereditary haemochromatosis. There is currently insufficient evidence to assess whether non-specific symptoms or hepatic injury in homozygotes with moderately elevated iron defined as a serum ferritin (SF) of 300-1000 µg/L are related to iron overload. As such the evidence for intervention in this group is lacking. We present here methods for a study that aims to evaluate whether non-specific symptoms and hepatic fibrosis markers improve with short-term normalisation of SF in p.C282Y homozygotes with moderate elevation of SF.
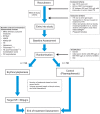
Methods and analysis: Mi-iron is a prospective, multicentre, randomised patient-blinded trial conducted in three centres in Victoria and Queensland, Australia. Participants who are HFE p.C282Y homozygotes with SF levels between 300 and 1000 μg/L are recruited and randomised to either the treatment group or to the sham treatment group. Those in the treatment group have normalisation of SF by 3-weekly erythrocytapheresis while those in the sham treatment group have 3-weekly plasmapheresis and thus do not have normalisation of SF. Patients are blinded to all procedures. All outcome measures are administered prior to and following the course of treatment/sham treatment. Patient reported outcome measures are the Modified Fatigue Impact Scale (MFIS-primary outcome), Hospital Anxiety and Depression Scale (HADS), Medical Outcomes Study 36-item short form V.2 (SF36v2) and Arthritis Impact Measurement Scale 2 short form (AIMS2-SF). Liver injury and hepatic fibrosis are assessed with transient elastography (TE), Fibrometer and Hepascore, while oxidative stress is assessed by measurement of urine and serum F2-isoprostanes.
Ethics and dissemination: This study has been approved by the Human Research Ethics Committees of Austin Health, Royal Melbourne Hospital and Royal Brisbane and Women's Hospital. Study findings will be disseminated through peer-reviewed publications and conference presentations.
Trial registration: Trial identifier: NCT01631708; Registry: ClinicalTrials.gov.
Keywords: erythrocytapheresis; ferritin; haemochromatosis; phlebotomy; venesection.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


References
-
- Adams PC, Speechley M, Kertesz AE. Long-term survival analysis in hereditary hemochromatosis. Gastroeneterology 1991;101:368–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
