Estradiol regulates human QT-interval: acceleration of cardiac repolarization by enhanced KCNH2 membrane trafficking
- PMID: 26271031
- PMCID: PMC4753025
- DOI: 10.1093/eurheartj/ehv371
Estradiol regulates human QT-interval: acceleration of cardiac repolarization by enhanced KCNH2 membrane trafficking
Abstract
Background: Modulation of cardiac repolarization by sexual hormones is controversial and hormonal effects on ion channels remain largely unknown. In the present translational study, we therefore assessed the relationship between QTc duration and gonadal hormones and studied underlying mechanisms.
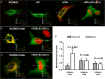
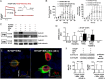
Methods and results: We measured hormone levels and QTc intervals in women during clomiphene stimulation for infertility and women before, during, and after pregnancy. Three heterozygous LQT-2 patients (KCNH2-p.Arg752Pro missense mutation) and two unaffected family members additionally were studied during their menstrual cycles. A comprehensive cellular and molecular analysis was done to identify the mechanisms of hormonal QT-interval regulation. High estradiol levels, but neither progesterone nor estradiol/progesterone ratio, inversely correlated with QTc. Consistent with clinical data, in vitro estradiol stimulation (60 pmol/L, 48 h) enhanced IKCNH2. This increase was mediated by estradiol receptor-α-dependent promotion of KCNH2-channel trafficking to the cell membrane. To study the underlying mechanism, we focused on heat-shock proteins. The heat-shock protein-90 (Hsp90) inhibitor geldanamycin abolished estradiol-induced increase in IKCNH2. Geldanamycin had no effect on KCNH2 transcription or translation; nor did it affect expression of estradiol receptors and chaperones. Estradiol enhanced the physical interaction of KCNH2-channel subunits with heat-shock proteins and augmented ion-channel trafficking to the membrane.
Conclusion: Elevated estradiol levels were associated with shorter QTc intervals in healthy women and female LQT-2 patients. Estradiol acts on KCNH2 channels via enhanced estradiol-receptor-α-mediated Hsp90 interaction, augments membrane trafficking and thereby increases repolarizing current. These results provide mechanistic insights into hormonal control of human ventricular repolarization and open novel therapeutic avenues.
Keywords: Gender; Hormones; Ion channels; Long-QT syndrome; Repolarization.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2015. For permissions please email: journals.permissions@oup.com.
Figures






Comment in
-
Another jigsaw piece in the complex picture of hormonal regulation of cardiac repolarization.Eur Heart J. 2016 Feb 14;37(7):651-3. doi: 10.1093/eurheartj/ehv475. Epub 2015 Sep 20. Eur Heart J. 2016. PMID: 26392434 No abstract available.
References
-
- Bazett HC. An analysis of the time relationship of electrocardiograms. Heart 1920;7:353–370.
-
- Sauer AJ, Moss AJ, McNitt S, Peterson DR, Zareba W, Robinson JL, Qi M, Goldenberg I, Hobbs JB, Ackerman MJ, Benhorin J, Hall WJ, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long QT syndrome in adults. J Am Coll Cardiol 2007;49:329–337. - PubMed
-
- Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ Res 2003;92:e87–100. - PubMed
-
- Pham TV, Rosen MR. Sex, hormones, and repolarization. Cardiovasc Res 2002;53:740–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

