Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer
- PMID: 26271155
- PMCID: PMC4993045
- DOI: 10.1016/j.ygyno.2015.08.004
Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer
Abstract
Objective: OCEANS is a randomized, placebo (PL)-controlled, phase 3 trial evaluating the efficacy and safety of bevacizumab combined with gemcitabine+carboplatin (GC) for patients with platinum-sensitive recurrent ovarian cancer (ROC). The study met its primary endpoint, demonstrating improved progression-free survival with GC+bevacizumab compared with GC+PL. Herein, we describe results of final overall survival (OS) and updated safety.
Methods: Patients with recurrent platinum-sensitive ROC (recurring ≥6months after first-line platinum-based therapy) and measurable disease at baseline were randomized to receive GC+bevacizumab or GC+PL for 6-10cycles; PL or bevacizumab was then continued until disease progression. In this updated analysis, a Cox proportional hazards model was used to compare OS between the 2 treatment arms.
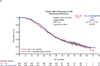
Results: At the data cutoff date (July 19, 2013), 353 patients (72.9%) had died. Median follow-up for OS was 58.2months in the experimental arm and 56.4months in the control arm. Consistent with interim analyses, median OS was comparable between arms (GC+bevacizumab: 33.6months; GC+PL: 32.9months; hazard ratio=0.95; log-rank p=0.65), and was consistent across all examined patient subgroups. The frequency and severity of adverse events were consistent with previous analyses; no new safety concerns were identified.
Conclusions: Results from final OS analysis of the phase 3 OCEANS study showed no significant difference in OS for patients treated with GC+bevacizumab compared with GC+PL.
Trial registration: ClinicalTrials.gov NCT00434642.
Keywords: Bevacizumab; OCEANS; Ovarian cancer; Overall survival; Safety.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
A.H. and Y.V.W. are employees of Genentech, Inc. A.H. also has a patent pending with Genentech, Inc. S.V.B. has served on an advisory board for and received fees from Genentech, Inc.
Figures


References
-
- American Cancer Society. Ovarian cancer. 2014 Available at: http://www.cancer.org/acs/groups/cid/documents/webcontent/003130-pdf.pdf. Last Medical Review: 08/05/2014; Last Revised: 12/23/2014.
-
- Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120:612–618. - PubMed
-
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with Stage III and Stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. - PubMed
-
- Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

