Role of the steroidogenic acute regulatory protein in health and disease
- PMID: 26271515
- PMCID: PMC4707056
- DOI: 10.1007/s12020-015-0715-6
Role of the steroidogenic acute regulatory protein in health and disease
Abstract
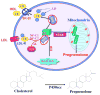
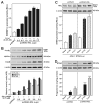
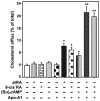
Steroid hormones are an important class of regulatory molecules that are synthesized in steroidogenic cells of the adrenal, ovary, testis, placenta, brain, and skin, and influence a spectrum of developmental and physiological processes. The steroidogenic acute regulatory protein (STAR) predominantly mediates the rate-limiting step in steroid biosynthesis, i.e., the transport of the substrate of all steroid hormones, cholesterol, from the outer to the inner mitochondrial membrane. At the inner membrane, cytochrome P450 cholesterol side chain cleavage enzyme cleaves the cholesterol side chain to form the first steroid, pregnenolone, which is converted by a series of enzymes to various steroid hormones in specific tissues. Both basic and clinical evidence have demonstrated the crucial involvement of the STAR protein in the regulation of steroid biosynthesis. Multiple levels of regulation impinge on STAR action. Recent findings demonstrate that hormone-sensitive lipase, through its action on the hydrolysis of cholesteryl esters, plays an important role in regulating STAR expression and steroidogenesis which involve the liver X receptor pathway. Activation of the latter influences macrophage cholesterol efflux that is a key process in the prevention of atherosclerotic cardiovascular disease. Appropriate regulation of steroid hormones is vital for proper functioning of many important biological activities, which are also paramount for geriatric populations to live longer and healthier. This review summarizes the current level of understanding on tissue-specific and hormone-induced regulation of STAR expression and steroidogenesis, and provides insights into a number of cholesterol and/or steroid coupled physiological and pathophysiological consequences.
Keywords: Aging; Atherosclerosis; HSL and LXR; STAR; STAR deficiency and Lipoid CAH; Steroidogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Azhar S, Reaven E. Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation of steroidogenesis. Mol Cell Endocrinol. 2002;195:1–26. - PubMed
-
- Kraemer FB, Shen WJ, Harada K, Patel S, Osuga J, Ishibashi S, Azhar S. Hormone-sensitive lipase is required for high-density lipoprotein cholesteryl ester-supported adrenal steroidogenesis. Mol Endocrinol. 2004;18:549–557. - PubMed
-
- Manna PR, Stocco DM. Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:93–108. - PubMed
-
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19:2647–2659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

