The Endocannabinoid System and its Modulation by Phytocannabinoids
- PMID: 26271952
- PMCID: PMC4604172
- DOI: 10.1007/s13311-015-0374-6
The Endocannabinoid System and its Modulation by Phytocannabinoids
Abstract
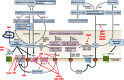
The endocannabinoid system is currently defined as the ensemble of the two 7-transmembrane-domain and G protein-coupled receptors for Δ(9)-tetrahydrocannabinol (but not for most other plant cannabinoids or phytocannabinoids)-cannabinoid receptor type-1 (CB1R) and cannabinoid receptor type-2 (CB2R); their two most studied endogenous ligands, the "endocannabinoids" N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol (2-AG); and the enzymes responsible for endocannabinoid metabolism. However, anandamide and 2-AG, and also the phytocannabinoids, have more molecular targets than just CB1R and CB2R. Furthermore, the endocannabinoids, like most other lipid mediators, have more than just one set of biosynthetic and degrading pathways and enzymes, which they often share with "endocannabinoid-like" mediators that may or may not interact with the same proteins as Δ(9)-tetrahydrocannabinol and other phytocannabinoids. In some cases, these degrading pathways and enzymes lead to molecules that are not inactive and instead interact with other receptors. Finally, some of the metabolic enzymes may also participate in the chemical modification of molecules that have very little to do with endocannabinoid and cannabinoid targets. Here, we review the whole world of ligands, receptors, and enzymes, a true "endocannabinoidome", discovered after the cloning of CB1R and CB2R and the identification of anandamide and 2-AG, and its interactions with phytocannabinoids.
Keywords: Endocannabinoidome; Endocannabinoids; Phytocannabinoids; TRP channels.
Figures
References
-
- Di Marzo V, De Petrocellis L. Fifty years of ‘cannabinoid research’ and the need for a new nomenclature. In: Di Marzo V (ed.) Cannabinoids, Wiley-Blackwell, Oxford, 2014, pp. 261–289.
-
- Di Marzo V, Wang J (eds). The endocannabinoidome. Academic Press, London, 2015.
-
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/S0014-5793(00)02082-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

