Early severe impairment of hematopoietic stem and progenitor cells from the bone marrow caused by CLP sepsis and endotoxemia in a humanized mice model
- PMID: 26272069
- PMCID: PMC4536694
- DOI: 10.1186/s13287-015-0135-9
Early severe impairment of hematopoietic stem and progenitor cells from the bone marrow caused by CLP sepsis and endotoxemia in a humanized mice model
Abstract
Introduction: An effective immune response to severe bacterial infections requires a robust production of the innate immunity cells from hematopoietic stem and progenitor cells (HSPCs) in a process called emergency myelopoiesis. In sepsis, an altered immune response that leads to a failure of bacterial clearance is often observed. In this study, we aimed to evaluate the impact of sepsis on human HSPCs in the bone marrow (BM) microenvironment of humanized mice subjected to acute endotoxemia and polymicrobial sepsis.
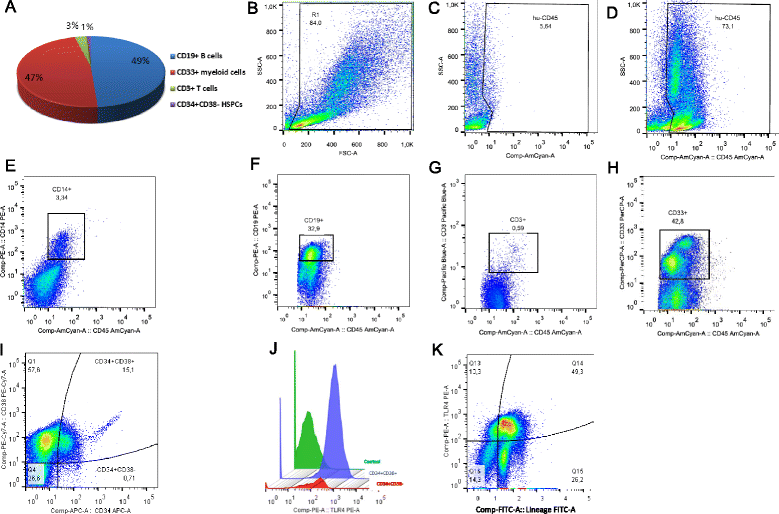
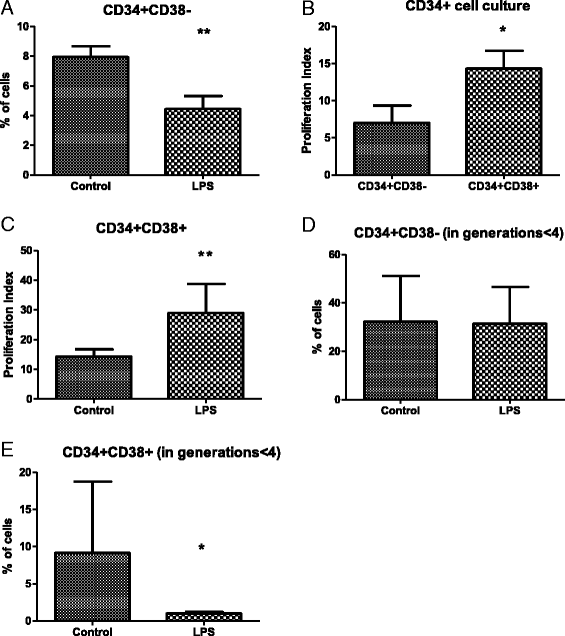
Methods: Humanized mice (hu-NSG) were generated by transplanting NOD.Cg-Prkdc/scidIL2rγ (NSG) mice with the human cord blood CD34(+) cells. Eight weeks after the transplantation, hu-NSG mice were subjected to sepsis induced by endotoxemia-Escherichia coli lipopolysaccharide (LPS)-or by cecal ligation and puncture (CLP). Twenty-four hours later, HSPCs from BM were analyzed by flow cytometry and colony-forming unit (CFU) assay. CLP after inhibition of Notch signaling was also performed. The effects of LPS on the in vitro proliferation of CD34(+) cells from human BM were tested by CellTrace Violet dye staining.
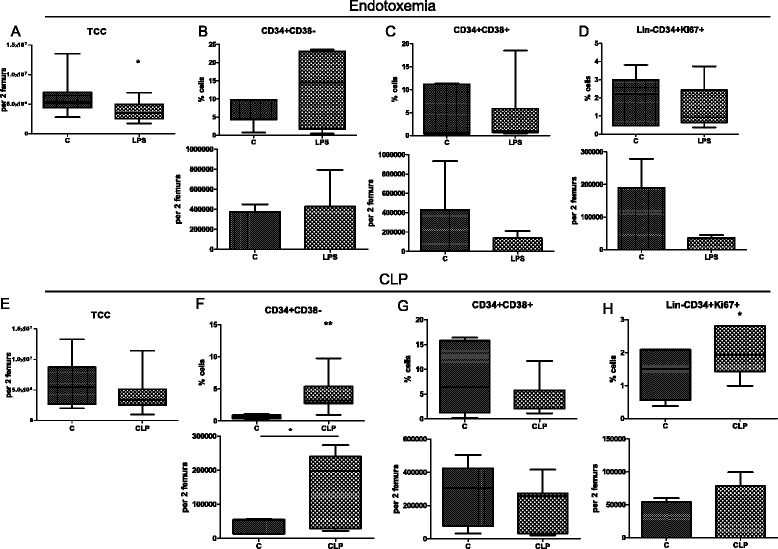
Results: The expression of Toll-like receptor 4 receptor was present among engrafted human HSPCs. Both CLP and endotoxemia decreased (by 43 % and 37 %) cellularity of the BM. In addition, in both models, accumulation of early CD34(+) CD38(-) HSCs was observed, but the number of CD34(+) CD38(+) progenitors decreased. After CLP, there was a 1.5-fold increase of proliferating CD34(+) CD38(-)Ki-67(+) cells. Moreover, CFU assay revealed a depressed (by 75 % after LPS and by 50 % after CLP) production of human hematopoietic colonies from the BM of septic mice. In contrast, in vitro LPS stimulated differentiation of CD34(+) CD38(-) HSCs but did not induce proliferation of these cells in contrast to the CD34(+) CD38(+) progenitors. CLP sepsis modulated the BM microenvironment by upregulation of Jagged-1 expression on non-hematopoietic cells, and the proliferation of HSCs was Notch-dependent.
Conclusions: CLP sepsis and endotoxemia induced a similar expansion and proliferation of early HSCs in the BM, while committed progenitors decreased. It is suggestive that the Notch pathway contributed to this effect. Targeting early hematopoiesis may be considered as a viable alternative in the existing arsenal of supportive therapies in sepsis.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

