Abnormalities of serotonergic neurotransmission in animal models of SUDEP
- PMID: 26272185
- PMCID: PMC4749463
- DOI: 10.1016/j.yebeh.2015.06.008
Abnormalities of serotonergic neurotransmission in animal models of SUDEP
Abstract
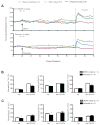
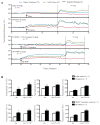
Sudden unexpected death in epilepsy (SUDEP) is a devastating event, and both DBA/1 and DBA/2 mice have been shown to be relevant animal models for studying SUDEP. DBA mice exhibit seizure-induced respiratory arrest (S-IRA), leading to cardiac arrest and subsequent sudden death after generalized audiogenic seizures (AGSs). This sequence of terminal events is also observed in the majority of witnessed human SUDEP cases. Several pathophysiological mechanisms, including respiratory/cardiac dysfunction, have been proposed to contribute to human SUDEP. Several (but not all) selective serotonin (5-HT) reuptake inhibitors (SSRIs), including fluoxetine, can reversibly block S-IRA, and abnormal expression of 5-HT receptors is found in the brainstem of DBA mice. DBA mice, which do not initially show S-IRA, exhibit S-IRA after treatment with a nonselective 5-HT antagonist. These studies suggest that abnormalities of 5-HT neurotransmission are involved in the pathogenesis of S-IRA in DBA mice. Serotonergic (5-HT) transmission plays an important role in normal respiration, and DBA mice exhibiting S-IRA can be resuscitated using a rodent ventilator. It is important and interesting to know if fluoxetine blocks S-IRA in DBA mice by enhancing respiratory ventilation. To test this, the effects of breathing stimulants, doxapram, and 5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine (PK-THPP) were compared with the effects of fluoxetine on S-IRA in DBA/1 mice. Although fluoxetine reduces the incidence of S-IRA in DBA/1 mice, as reported previously, the same dose of fluoxetine fails to enhance baseline respiratory ventilation in the absence of AGSs. Doxapram and PK-THPP augment the baseline ventilation in DBA/1 mice. However, these breathing stimulants are ineffective in preventing S-IRA in DBA/1 mice. These data suggest that fluoxetine blocks S-IRA in DBA/1 mice by cellular/molecular mechanisms other than enhancement of basal ventilation. Future research directions are also discussed. This article is part of a Special Issue entitled "Genetic and Reflex Epilepsies, Audiogenic Seizures and Strains: From Experimental Models to the Clinic".
Keywords: 5-HT receptors; DBA mice; Fluoxetine; Respiratory arrest; SSRI; Serotonin.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Hughes JR. A review of sudden unexpected death in epilepsy: prediction of patients at risk. Epilepsy Behav. 2009;14:280–7. - PubMed
-
- Nei M, Hays R. Sudden unexpected death in epilepsy. Curr Neurol Neurosci Rep. 2010;10:319–26. - PubMed
-
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–77. - PubMed
-
- Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: Assessing the public health burden. Epilepsia. 2014 - PubMed
-
- Stollberger C, Finsterer J. Cardiorespiratory findings in sudden unexplained/unexpected death in epilepsy (SUDEP) Epilepsy Res. 2004;59:51–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

