DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma
- PMID: 26272216
- PMCID: PMC4626662
- DOI: 10.1182/blood-2015-04-640912
DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma
Abstract
Follicular lymphoma (FL) results from the accumulation of malignant germinal center (GC) B cells leading to the development of an indolent and largely incurable disease. FL cells remain highly dependent on B-cell receptor (BCR) signaling and on a specific cell microenvironment, including T cells, macrophages, and stromal cells. Importantly, FL BCR is characterized by a selective pressure to retain surface immunoglobulin M (IgM) BCR despite an active class-switch recombination process, and by the introduction, in BCR variable regions, of N-glycosylation acceptor sites harboring unusual high-mannose oligosaccharides. However, the relevance of these 2 FL BCR features for lymphomagenesis remains unclear. In this study, we demonstrated that IgM(+) FL B cells activated a stronger BCR signaling network than IgG(+) FL B cells and normal GC B cells. BCR expression level and phosphatase activity could both contribute to such heterogeneity. Moreover, we underlined that a subset of IgM(+) FL samples, displaying highly mannosylated BCR, efficiently bound dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN), which could in turn trigger delayed but long-lasting BCR aggregation and activation. Interestingly, DC-SIGN was found within the FL cell niche in situ. Finally, M2 macrophages induced a DC-SIGN-dependent adhesion of highly mannosylated IgM(+) FL B cells and triggered BCR-associated kinase activation. Interestingly, pharmacologic BCR inhibitors abolished such crosstalk between macrophages and FL B cells. Altogether, our data support an important role for DC-SIGN-expressing infiltrating cells in the biology of FL and suggest that they could represent interesting therapeutic targets.
© 2015 by The American Society of Hematology.
Figures

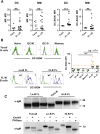
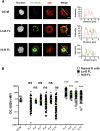
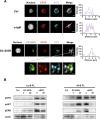
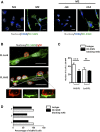
Comment in
-
Sugar-coated signaling in follicular lymphoma.Blood. 2015 Oct 15;126(16):1871-2. doi: 10.1182/blood-2015-08-665141. Blood. 2015. PMID: 26472734
References
-
- Amé-Thomas P, Tarte K. The yin and the yang of follicular lymphoma cell niches: role of microenvironment heterogeneity and plasticity. Semin Cancer Biol. 2014;24:23–32. - PubMed
-
- Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14(8):517–534. - PubMed
-
- Zuckerman NS, McCann KJ, Ottensmeier CH, et al. Ig gene diversification and selection in follicular lymphoma, diffuse large B cell lymphoma and primary central nervous system lymphoma revealed by lineage tree and mutation analyses. Int Immunol. 2010;22(11):875–887. - PubMed
-
- Loeffler M, Kreuz M, Haake A, et al. HaematoSys-Project. Genomic and epigenomic co-evolution in follicular lymphomas. Leukemia. 2015;29(2):456–463. - PubMed
-
- Meeker T, Lowder J, Cleary ML, et al. Emergence of idiotype variants during treatment of B-cell lymphoma with anti-idiotype antibodies. N Engl J Med. 1985;312(26):1658–1665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

