Staphylococcal manipulation of host immune responses
- PMID: 26272408
- PMCID: PMC4625792
- DOI: 10.1038/nrmicro3521
Staphylococcal manipulation of host immune responses
Abstract
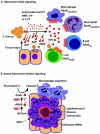
Staphylococcus aureus, a bacterial commensal of the human nares and skin, is a frequent cause of soft tissue and bloodstream infections. A hallmark of staphylococcal infections is their frequent recurrence, even when treated with antibiotics and surgical intervention, which demonstrates the bacterium's ability to manipulate innate and adaptive immune responses. In this Review, we highlight how S. aureus virulence factors inhibit complement activation, block and destroy phagocytic cells and modify host B cell and T cell responses, and we discuss how these insights might be useful for the development of novel therapies against infections with antibiotic resistant strains such as methicillin-resistant S. aureus.
Figures



References
-
- van Belkum A, et al. Co-evolutionary aspects of human colonisation and infection by Staphylococcus aureus. Infect. Genet. Evol. 2009;9:32–47. - PubMed
-
- Kallen AJ, et al. Health care-associated invasive MRSA infections, 2005-2008. JAMA. 2010;304:641–648. - PubMed
-
- Spaan AN, Surewaard BGJ, Nijland R, van Strijp JAG. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu. Rev. Microbiol. 2013;67:629–650. This excellent review summarizes the molecular events that occur during encounters between neutrophils and staphylococci. - PubMed
-
- Curnutte JT, Whitten DM, Babior BM. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N. Engl. J. Med. 1974;290:593–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

