Catalysis of GTP hydrolysis by small GTPases at atomic detail by integration of X-ray crystallography, experimental, and theoretical IR spectroscopy
- PMID: 26272610
- PMCID: PMC4591799
- DOI: 10.1074/jbc.M115.648071
Catalysis of GTP hydrolysis by small GTPases at atomic detail by integration of X-ray crystallography, experimental, and theoretical IR spectroscopy
Abstract
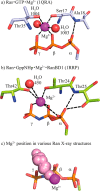
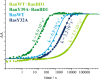
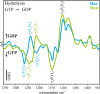
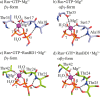
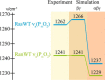
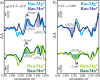
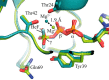
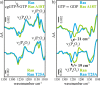
Small GTPases regulate key processes in cells. Malfunction of their GTPase reaction by mutations is involved in severe diseases. Here, we compare the GTPase reaction of the slower hydrolyzing GTPase Ran with Ras. By combination of time-resolved FTIR difference spectroscopy and QM/MM simulations we elucidate that the Mg(2+) coordination by the phosphate groups, which varies largely among the x-ray structures, is the same for Ran and Ras. A new x-ray structure of a Ran·RanBD1 complex with improved resolution confirmed this finding and revealed a general problem with the refinement of Mg(2+) in GTPases. The Mg(2+) coordination is not responsible for the much slower GTPase reaction of Ran. Instead, the location of the Tyr-39 side chain of Ran between the γ-phosphate and Gln-69 prevents the optimal positioning of the attacking water molecule by the Gln-69 relative to the γ-phosphate. This is confirmed in the RanY39A·RanBD1 crystal structure. The QM/MM simulations provide IR spectra of the catalytic center, which agree very nicely with the experimental ones. The combination of both methods can correlate spectra with structure at atomic detail. For example the FTIR difference spectra of RasA18T and RanT25A mutants show that spectral differences are mainly due to the hydrogen bond of Thr-25 to the α-phosphate in Ran. By integration of x-ray structure analysis, experimental, and theoretical IR spectroscopy the catalytic center of the x-ray structural models are further refined to sub-Å resolution, allowing an improved understanding of catalysis.
Keywords: Fourier transform IR (FTIR); QM/MM simulations; X-ray crystallography; computer modeling; crystal structure; infrared spectroscopy (IR spectroscopy); nuclear transport; reaction mechanism; small GTPase; spectroscopy.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Wittinghofer A., Vetter I. R. (2011) Structure-function relationships of the G domain, a canonical switch motif. Annu. Rev. Biochem. 80, 943–971 - PubMed
-
- Cherfils J., Zeghouf M. (2013) Regulation of Small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 - PubMed
-
- Ligeti E., Welti S., Scheffzek K. (2012) Inhibition and termination of physiological responses by GTPase activating proteins. Physiol. Rev. 92, 237–272 - PubMed
-
- Stewart M. (2007) Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell Biol. 8, 195–208 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

