Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix
- PMID: 26272817
- PMCID: PMC4539566
- DOI: 10.1038/ncomms9026
Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix
Abstract
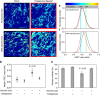
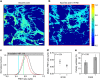
Despite the crucial role of extracellular matrix (ECM) in directing cell fate in healthy and diseased tissues--particularly in development, wound healing, tissue regeneration and cancer--the mechanisms that direct the assembly and regulate hierarchical architectures of ECM are poorly understood. Collagen I matrix assembly in vivo requires active fibronectin (Fn) fibrillogenesis by cells. Here we exploit Fn-FRET probes as mechanical strain sensors and demonstrate that collagen I fibres preferentially co-localize with more-relaxed Fn fibrils in the ECM of fibroblasts in cell culture. Fibre stretch-assay studies reveal that collagen I's Fn-binding domain is responsible for the mechano-regulated interaction. Furthermore, we show that Fn-collagen interactions are reciprocal: relaxed Fn fibrils act as multivalent templates for collagen assembly, but once assembled, collagen fibres shield Fn fibres from being stretched by cellular traction forces. Thus, in addition to the well-recognized, force-regulated, cell-matrix interactions, forces also tune the interactions between different structural ECM components.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

