Photosynthetic lesions can trigger accelerated senescence in Arabidopsis thaliana
- PMID: 26272903
- PMCID: PMC4623695
- DOI: 10.1093/jxb/erv393
Photosynthetic lesions can trigger accelerated senescence in Arabidopsis thaliana
Abstract
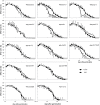
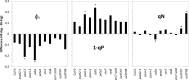
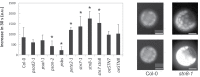
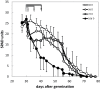
Senescence is a highly regulated process characterized by the active breakdown of cells, which ultimately leads to the death of plant organs or whole plants. In annual plants such as Arabidopsis thaliana senescence can be observed in each individual leaf. Whether deficiencies in photosynthesis promote the induction of senescence was investigated by monitoring chlorophyll degradation, photosynthetic parameters, and reactive oxygen species accumulation in photosynthetic mutants. Several mutations affecting components of the photosynthetic apparatus, including psal-2, psan-2, and psbs, were found to lead to premature or faster senescence, as did simultaneous inactivation of the STN7 and STN8 kinases. Premature senescence is apparently not directly linked to an overall reduction in photosynthesis but to perturbations in specific aspects of the process. Dark-induced senescence is accelerated in mutants affected in linear electron flow, especially psad2-1, psan-2, and pete2-1, as well as in stn7 and stn8 mutants and STN7 and STN8 overexpressor lines. Interestingly, no direct link with ROS production could be observed.
Keywords: Arabidopsis; ROS; STN7; STN8.; photosynthesis; photosystem; senescence.
© The Author 2015. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures

References
-
- Armbruster U, Rühle T, Kreller R, Strotbek C, Zühlke J, Tadini L, Blunder T, Hertle AP, Qi Y, Rengstl B, Nickelsen J, Frank W, Leister D. 2013. The photosynthesis affected mutant68-like protein evolved from a PSII assembly factor to mediate assembly of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis . The Plant Cell 25, 3926–3943. - PMC - PubMed
-
- Arrom L, Munne-Bosch S. 2012. Hormonal regulation of leaf senescence in Lilium . Journal of Plant Physiology 169, 1542–1550. - PubMed
-
- Bellafiore S, Barneche F, Peltier G, Rochaix JD. 2005. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

