Increased Adipocyte Area in Injured Muscle With Aging and Impaired Remodeling in Female Mice
- PMID: 26273023
- PMCID: PMC4945881
- DOI: 10.1093/gerona/glv104
Increased Adipocyte Area in Injured Muscle With Aging and Impaired Remodeling in Female Mice
Abstract
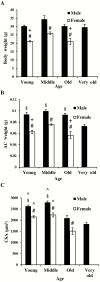
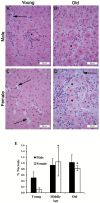
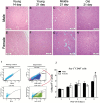
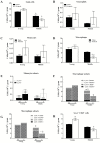
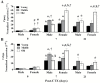
We demonstrated that young male and female mice similarly regenerated injured skeletal muscle; however, female mice transiently increased adipocyte area within regenerated muscle in a sex hormone-dependent manner. We extended these observations to investigate the effect of aging and sex on sarcopenia and muscle regeneration. Cardiotoxin injury to the tibialis anterior muscle of young, middle, and old-aged C57Bl/6J male and female mice was used to measure regenerated myofiber cross-sectional area (CSA), adipocyte area, residual necrosis, and inflammatory cell recruitment. Baseline (uninjured) myofiber CSA was decreased in old mice of both sexes compared to young and middle-aged mice. Regenerated CSA was similar in male mice in all age groups until baseline CSA was attained but decreased in middle and old age female mice compared to young females. Furthermore, adipocyte area within regenerated muscle was transiently increased in young females compared to young males and these sex-dependent increases persisted in middle and old age female mice and were associated with increased Pparg Young female mice had more pro-inflammatory monocytes/macrophages in regenerating muscle than young male mice and increased Sca-1(+)CD45(-)cells. In conclusion, sex and age influence pro-inflammatory cell recruitment, muscle regeneration, and adipocyte area following skeletal muscle injury.
Keywords: Monocyte/macrophage; Muscle regeneration; Sarcopenia.
© The Author 2015. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf). 2008;194:81–93. doi:10.1111/j.1748-1716.2008.01861.x - PubMed
-
- Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol (1985). 2008;104:347–353. doi:10.1152/japplphysiol.00128.2007 - PubMed
-
- Sherwood RI, Christensen JL, Conboy IM, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi:http://dx.doi.org/10.1016/j.cell.2004.10.021 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

