Modulation of Hyaluronan Synthesis by the Interaction between Mesenchymal Stem Cells and Osteoarthritic Chondrocytes
- PMID: 26273306
- PMCID: PMC4529975
- DOI: 10.1155/2015/640218
Modulation of Hyaluronan Synthesis by the Interaction between Mesenchymal Stem Cells and Osteoarthritic Chondrocytes
Abstract
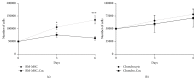
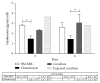
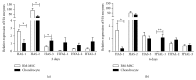
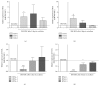
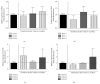
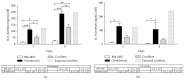
Bone marrow mesenchymal stem cells (BM-MSCs) are considered a good source for cellular therapy in cartilage repair. But, their potential to repair the extracellular matrix, in an osteoarthritic environment, is still controversial. In osteoarthritis (OA), anti-inflammatory action and extracellular matrix production are important steps for cartilage healing. This study examined the interaction of BM-MSC and OA-chondrocyte on the production of hyaluronan and inflammatory cytokines in a Transwell system. We compared cocultured BM-MSCs and OA-chondrocytes with the individually cultured controls (monocultures). There was a decrease in BM-MSCs cell count in coculture with OA-chondrocytes when compared to BM-MSCs alone. In monoculture, BM-MSCs produced higher amounts of hyaluronan than OA-chondrocytes and coculture of BM-MSCs with OA-chondrocytes increased hyaluronan production per cell. Hyaluronan synthase-1 mRNA expression was upregulated in BM-MSCs after coculture with OA-chondrocytes, whereas hyaluronidase-1 was downregulated. After coculture, lower IL-6 levels were detected in BM-MSCs compared with OA-chondrocytes. These results indicate that, in response to coculture with OA-chondrocytes, BM-MSCs change their behavior by increasing production of hyaluronan and decreasing inflammatory cytokines. Our results indicate that BM-MSCs per se could be a potential tool for OA regenerative therapy, exerting short-term effects on the local microenvironment even when cell:cell contact is not occurring.
Figures
Similar articles
-
Fibroblast growth factor 18 increases the trophic effects of bone marrow mesenchymal stem cells on chondrocytes isolated from late stage osteoarthritic patients.Stem Cells Int. 2014;2014:125683. doi: 10.1155/2014/125683. Epub 2014 Dec 3. Stem Cells Int. 2014. PMID: 25544847 Free PMC article.
-
Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis.J Cell Mol Med. 2021 Oct;25(19):9281-9294. doi: 10.1111/jcmm.16860. Epub 2021 Aug 27. J Cell Mol Med. 2021. PMID: 34448527 Free PMC article.
-
Adeno-Associated Virus-Mediated Overexpression of Interleukin-10 Affects the Immunomodulatory Properties of Equine Bone Marrow-Derived Mesenchymal Stem Cells.Hum Gene Ther. 2021 Sep;32(17-18):907-918. doi: 10.1089/hum.2020.319. Epub 2021 May 14. Hum Gene Ther. 2021. PMID: 33843261
-
Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration.Methods. 2016 Apr 15;99:69-80. doi: 10.1016/j.ymeth.2015.09.015. Epub 2015 Sep 15. Methods. 2016. PMID: 26384579 Review.
-
Effects of Immune Cells and Cytokines on Different Cells in OA.J Inflamm Res. 2023 May 30;16:2329-2343. doi: 10.2147/JIR.S413578. eCollection 2023. J Inflamm Res. 2023. PMID: 37273484 Free PMC article. Review.
Cited by
-
Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis.Stem Cell Res Ther. 2022 Jan 10;13(1):14. doi: 10.1186/s13287-021-02689-9. Stem Cell Res Ther. 2022. PMID: 35012666 Free PMC article. Review.
-
The U3 and Env Proteins of Jaagsiekte Sheep Retrovirus and Enzootic Nasal Tumor Virus Both Contribute to Tissue Tropism.Viruses. 2019 Nov 14;11(11):1061. doi: 10.3390/v11111061. Viruses. 2019. PMID: 31739606 Free PMC article.
-
Role of Inflammatory Niche and Adult Cardiomyocyte Coculture on Differentiation, Matrix Synthesis, and Secretome Release by Human Bone Marrow Mesenchymal Stem Cells.Appl Biochem Biotechnol. 2022 May;194(5):1938-1954. doi: 10.1007/s12010-022-03803-0. Epub 2022 Jan 8. Appl Biochem Biotechnol. 2022. PMID: 35000124
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

