Apoptosis induction by combination of drugs or a conjugated molecule associating non-steroidal anti-inflammatory and nitric oxide donor effects in medullary thyroid cancer models: implication of the tumor suppressor p73
- PMID: 26273323
- PMCID: PMC4535850
- DOI: 10.1186/s13044-015-0025-3
Apoptosis induction by combination of drugs or a conjugated molecule associating non-steroidal anti-inflammatory and nitric oxide donor effects in medullary thyroid cancer models: implication of the tumor suppressor p73
Abstract
Background: Medullary thyroid cancer (MTC) is a C-cell neoplasm. Surgery remains its main treatment. Promising therapies based on tyrosine kinase inhibitors demand careful patient selection. We previously observed that two non-steroidal anti-inflammatory drugs (NSAID), indomethacin, celecoxib, and nitric oxide (NO) prevented tumor growth in a model of human MTC cell line (TT) in nude mice.
Methods: In the present study, we tested the NO donor: glyceryl trinitrate (GTN), at pharmacological dose, alone and in combination with each of the two NSAIDs on TT cells. We also assessed the anti-proliferative potential of NO-indomethacin, an indomethacin molecule chemically conjugated with a NO moiety (NCX 530, Nicox SA) on TT cells and indomethacin/GTN association in rMTC 6-23 cells. The anti-tumoral action of the combined sc. injections of GTN with oral delivery of indomethacin was also studied on subcutaneous TT tumors in nude mice. Apoptosis mechanisms were assessed by expression of caspase-3, TAp73α, TAp73α inhibition by siRNA or Annexin V externalisation.
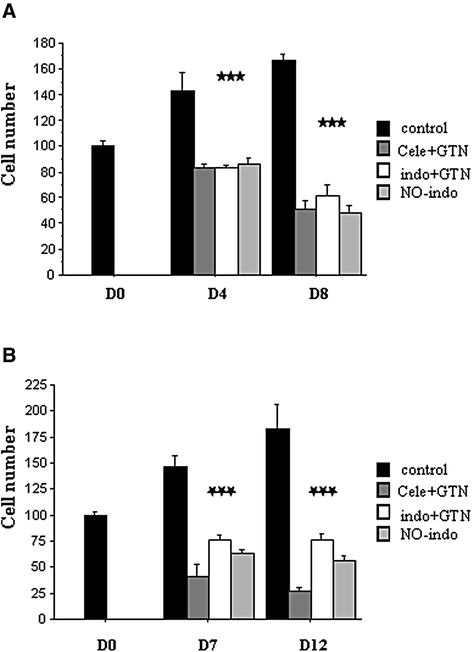
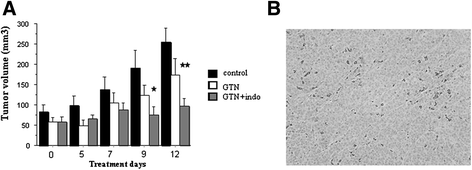
Results: The two NSAIDs and GTN reduced mitotic activity in TT cells versus control (cell number and PCNA protein expression). The combined treatments amplified the anti-tumor effect of single agents in the two tested cell lines and promoted cell death. Moreover, indomethacin/GTN association stopped the growth of established TT tumors in nude mice. We observed a significant cleavage of full length PARP, a caspase-3 substrate. The cell death appearance was correlated with a two-fold increase in TAp73α expression, with inhibition of apoptosis after TAp73α siRNA addition, demonstrating its crucial role in apoptosis.
Conclusion: Association of NO with NSAID exhibited amplified anti-tumoral effects on in vitro and in vivo MTC models by inducing p73-dependent apoptotic cell death.
Keywords: Apoptosis; Medullary thyroid carcinoma; NO-donors; Non-steroidal anti-inflammatory drugs; TT cells; rMTC 6–23 cells.
Figures




References
-
- Cohen R, Campos J, Salaün C, Heshmati H, Kraimps J, Proye C, Sarfati E, Henry J, Niccoli-Sire P, Modigliani E. Preoperative calcitonin levels are predictive of tumor size and postoperative calcitonin normalization in medullary thyroid carcinoma. J Clin Endocrinol Metab. 2000;85:919–22. doi: 10.1210/jcem.85.2.6556. - DOI - PubMed
-
- Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG. Revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

