Endogenous Glucocorticoids and Bone
- PMID: 26273496
- PMCID: PMC4472112
- DOI: 10.4248/BR201302001
Endogenous Glucocorticoids and Bone
Abstract
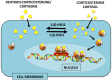
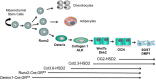
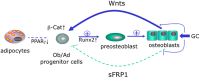
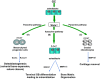
While the adverse effects of glucocorticoids on bone are well described, positive effects of glucocorticoids on the differentiation of osteoblasts are also observed. These paradoxical effects of glucocorticoids are dose dependent. At both physiologicaland supraphysiological levels of glucocorticoids, osteoblasts and osteocytes are the major glucocorticoid target cells. However, the response of the osteoblasts to each of these is quite distinct. At physiology levels, glucocorticoids direct mesenchymal progenitor cells to differentiate towards osteoblasts and thus increase bone formation in a positive way. In contrast with ageing, the excess production of glucocorticoids, at both systemic and intracellular levels, appear to impact on osteoblast and osteocytes in a negative way in a similar fashion to that seen with therapeutic glucocorticoids. This review will focus on therole of glucocorticoids in normal bone physiology, with particular emphasis on the mechanism by which endogenous glucocorticoids impact on bone and its constituent cells.
Keywords: Wnt signaling; bone; glucocorticoids; mechanisms of action; osteoblasts; skeletal development.
Figures
References
-
- Hench PS, Kendall EC. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949;24:181–197. - PubMed
-
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23 1:99–103. - PubMed
-
- Adinoff AD, Hollister JR. Steroid-induced fractures and bone loss in patients with asthma. N Engl J Med. 1983;309:265–268. - PubMed
-
- Turner RT, Hannon KS, Greene VS, Bell NH. Prednisone inhibits formation of cortical bone in sham-operated and ovariectomized female rats. Calcif Tissue Int. 1995;56:311–315. - PubMed
-
- Altman A, Hochberg Z, Silbermann M. Interactions between growth hormone and dexamethasone in skeletal growth and bone structure of the young mouse. Calcif Tissue Int. 1992;51:298–304. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

