Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective
- PMID: 26273505
- PMCID: PMC4472104
- DOI: 10.4248/BR201303002
Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective
Abstract
The role of Bone Tissue Engineering in the field of Regenerative Medicine has been the topic of substantial research over the past two decades. Technological advances have improved orthopaedic implants and surgical techniques for bone reconstruction. However, improvements in surgical techniques to reconstruct bone have been limited by the paucity of autologous materials available and donor site morbidity. Recent advances in the development of biomaterials have provided attractive alternatives to bone grafting expanding the surgical options for restoring the form and function of injured bone. Specifically, novel bioactive (second generation) biomaterials have been developed that are characterised by controlled action and reaction to the host tissue environment, whilst exhibiting controlled chemical breakdown and resorption with an ultimate replacement by regenerating tissue. Future generations of biomaterials (third generation) are designed to be not only osteoconductive but also osteoinductive, i.e. to stimulate regeneration of host tissues by combining tissue engineering and in situ tissue regeneration methods with a focus on novel applications. These techniques will lead to novel possibilities for tissue regeneration and repair. At present, tissue engineered constructs that may find future use as bone grafts for complex skeletal defects, whether from post-traumatic, degenerative, neoplastic or congenital/developmental "origin" require osseous reconstruction to ensure structural and functional integrity. Engineering functional bone using combinations of cells, scaffolds and bioactive factors is a promising strategy and a particular feature for future development in the area of hybrid materials which are able to exhibit suitable biomimetic and mechanical properties. This review will discuss the state of the art in this field and what we can expect from future generations of bone regeneration concepts.
Keywords: additve manufacturing; bone tissue engineering; clinical translation; regenerative medicine; scaffolds.
Figures

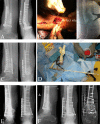




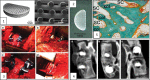
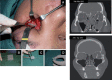

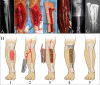
References
-
- Mason C. Regenerative medicine 2.0. Regen Med. 2007;2:11–18. - PubMed
-
- Webster TJ, Ahn ES. Nanostructured biomaterials for tissue engineering bone. Adv Biochem Eng Biotechnol. 2007;103:275–308. - PubMed
-
- Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20:92–102. - PubMed
-
- Keaveny TM, Morgan EF, Niebur GL, Yeh OC. Biomechanics of trabecular bone. Annu Rev Biomed Eng. 2001;3:307–333. - PubMed
-
- Barrère F, Mahmood TA, De Groot K, van Blitterswijk CA. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mat Sci Eng R. 2008;59:38–71.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

