Skeletal Blood Flow in Bone Repair and Maintenance
- PMID: 26273509
- PMCID: PMC4472118
- DOI: 10.4248/BR201304002
Skeletal Blood Flow in Bone Repair and Maintenance
Abstract
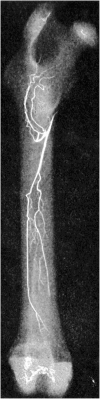
Bone is a highly vascularized tissue, although this aspect of bone is often overlooked. In this article, the importance of blood flow in bone repair and regeneration will be reviewed. First, the skeletal vascular anatomy, with an emphasis on long bones, the distinct mechanisms for vascularizing bone tissue, and methods for remodeling existing vasculature are discussed. Next, techniques for quantifying bone blood flow are briefly summarized. Finally, the body of experimental work that demonstrates the role of bone blood flow in fracture healing, distraction osteogenesis, osteoporosis, disuse osteopenia, and bone grafting is examined. These results illustrate that adequate bone blood flow is an important clinical consideration, particularly during bone regeneration and in at-risk patient groups.
Keywords: angiogenesis; blood flow; bone repair; fracture; vascular remodeling.
Figures


References
-
- Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res. 2006;21:183–192. - PubMed
-
- McCarthy I. The physiology of bone blood flow: a review. J Bone Joint Surg Am. 2006;88 Suppl 3:4–9. - PubMed
-
- Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol. 2012;8:358–366. - PubMed
-
- Burkhardt R, Kettner G, Bohm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–164. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

