Comprehensive Analysis of the 16p11.2 Deletion and Null Cntnap2 Mouse Models of Autism Spectrum Disorder
- PMID: 26273832
- PMCID: PMC4537259
- DOI: 10.1371/journal.pone.0134572
Comprehensive Analysis of the 16p11.2 Deletion and Null Cntnap2 Mouse Models of Autism Spectrum Disorder
Abstract
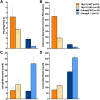
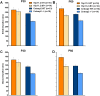
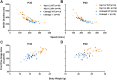
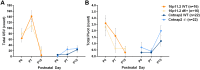
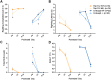
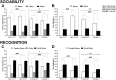
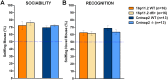
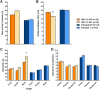
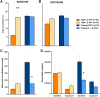
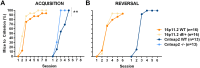
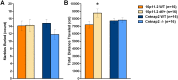
Autism spectrum disorder comprises several neurodevelopmental conditions presenting symptoms in social communication and restricted, repetitive behaviors. A major roadblock for drug development for autism is the lack of robust behavioral signatures predictive of clinical efficacy. To address this issue, we further characterized, in a uniform and rigorous way, mouse models of autism that are of interest because of their construct validity and wide availability to the scientific community. We implemented a broad behavioral battery that included but was not restricted to core autism domains, with the goal of identifying robust, reliable phenotypes amenable for further testing. Here we describe comprehensive findings from two known mouse models of autism, obtained at different developmental stages, using a systematic behavioral test battery combining standard tests as well as novel, quantitative, computer-vision based systems. The first mouse model recapitulates a deletion in human chromosome 16p11.2, found in 1% of individuals with autism. The second mouse model harbors homozygous null mutations in Cntnap2, associated with autism and Pitt-Hopkins-like syndrome. Consistent with previous results, 16p11.2 heterozygous null mice, also known as Del(7Slx1b-Sept1)4Aam weighed less than wild type littermates displayed hyperactivity and no social deficits. Cntnap2 homozygous null mice were also hyperactive, froze less during testing, showed a mild gait phenotype and deficits in the three-chamber social preference test, although less robust than previously published. In the open field test with exposure to urine of an estrous female, however, the Cntnap2 null mice showed reduced vocalizations. In addition, Cntnap2 null mice performed slightly better in a cognitive procedural learning test. Although finding and replicating robust behavioral phenotypes in animal models is a challenging task, such functional readouts remain important in the development of therapeutics and we anticipate both our positive and negative findings will be utilized as a resource for the broader scientific community.
Conflict of interest statement
Figures




References
-
- Menalled L, El-Khodor BF, Patry M, Suarez-Farinas M, Orenstein SJ, Zahasky B, et al. Systematic behavioral evaluation of Huntington's disease transgenic and knock-in mouse models. Neurobiol Dis. 2009;35(3):319–36. Epub 2009/05/26. S0969-9961(09)00107-7 [pii] 10.1016/j.nbd.2009.05.007 - DOI - PMC - PubMed
-
- Consortium TD-BFX. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78(1):23–33. Epub 1994/07/15. 0092-8674(94)90569-X [pii]. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

