Advances in Antibody Design
- PMID: 26274600
- PMCID: PMC5289076
- DOI: 10.1146/annurev-bioeng-071114-040733
Advances in Antibody Design
Abstract
The use of monoclonal antibodies as therapeutics requires optimizing several of their key attributes. These include binding affinity and specificity, folding stability, solubility, pharmacokinetics, effector functions, and compatibility with the attachment of additional antibody domains (bispecific antibodies) and cytotoxic drugs (antibody-drug conjugates). Addressing these and other challenges requires the use of systematic design methods that complement powerful immunization and in vitro screening methods. We review advances in designing the binding loops, scaffolds, domain interfaces, constant regions, post-translational and chemical modifications, and bispecific architectures of antibodies and fragments thereof to improve their bioactivity. We also highlight unmet challenges in antibody design that must be overcome to generate potent antibody therapeutics.
Keywords: CDR; Fab; IgG; VH; complementarity-determining region; scFv.
Figures
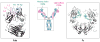

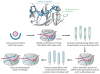

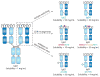



References
-
- Gitlin D. Current aspects of the structure, function, and genetics of the immunoglobulins. Annu Rev Med. 1966;17:1–22. - PubMed
-
- Lowe D, Dudgeon K, Rouet R, Schofield P, Jermutus L, Christ D. Aggregation, stability, and formulation of human antibody therapeutics. Adv Protein Chem Struct Biol. 2011;84:41–61. - PubMed
-
- Perchiacca JM, Tessier PM. Engineering aggregation-resistant antibodies. Annu Rev Chem Biomol Eng. 2012;3:263–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

