Osteopontin Deficiency Accelerates Spontaneous Colitis in Mice with Disrupted Gut Microbiota and Macrophage Phagocytic Activity
- PMID: 26274807
- PMCID: PMC4537118
- DOI: 10.1371/journal.pone.0135552
Osteopontin Deficiency Accelerates Spontaneous Colitis in Mice with Disrupted Gut Microbiota and Macrophage Phagocytic Activity
Abstract
Background: Osteopontin (OPN) is a multifunctional protein expressed in a variety of tissues and cells. Recent studies revealed increased OPN expression in the inflamed intestinal tissues of patients with inflammatory bowel disease (IBD). The role of OPN in the pathophysiology of IBD, however, remains unclear.
Aims: To investigate the role of OPN in the development of intestinal inflammation using a murine model of IBD, interleukin-10 knock out (IL-10 KO) mice.
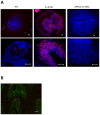
Methods: We compared the development of colitis between IL-10 KO and OPN/IL-10 double KO (DKO) mice. OPN expression in the colonic tissues of IL-10 KO mice was examined by fluorescence in situ hybridization (FISH) analysis. Enteric microbiota were compared between IL-10 KO and OPN/IL-10 DKO mice by terminal restriction fragment length polymorphism analysis. The effect of OPN on macrophage phagocytic function was evaluated by phagocytosis assay.
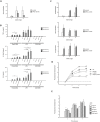
Results: OPN/IL-10 DKO mice had an accelerated onset of colitis compared to IL-10 KO mice. FISH analysis revealed enhanced OPN synthesis in the colonic epithelial cells of IL-10 KO mice. OPN/IL-10 DKO mice had a distinctly different enteric bacterial profile with a significantly lower abundance of Clostridium subcluster XIVa and a greater abundance of Clostridium cluster XVIII compared to IL-10 KO mice. Intracellular OPN deletion in macrophages impaired phagocytosis of fluorescence particle-conjugated Escherichia coli in vitro. Exogenous OPN enhanced phagocytosis by OPN-deleted macrophages when administered at doses of 1 to 100 ng/ml, but not 1000 ng/ml.
Conclusions: OPN deficiency accelerated the spontaneous development of colitis in mice with disrupted gut microbiota and macrophage phagocytic activity.
Conflict of interest statement
Figures


References
-
- Rittling SR (2011) Osteopontin in macrophage function. Expert Rev Mol Med 26:13. - PubMed
-
- Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CA, et al. (2004) Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol 198: 155–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

