Comparative Efficacy and Safety of Different Antiplatelet Agents for Prevention of Major Cardiovascular Events and Leg Amputations in Patients with Peripheral Arterial Disease: A Systematic Review and Network Meta-Analysis
- PMID: 26274912
- PMCID: PMC4537264
- DOI: 10.1371/journal.pone.0135692
Comparative Efficacy and Safety of Different Antiplatelet Agents for Prevention of Major Cardiovascular Events and Leg Amputations in Patients with Peripheral Arterial Disease: A Systematic Review and Network Meta-Analysis
Abstract
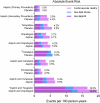
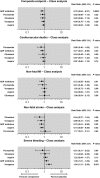
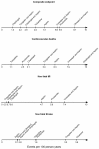
There is a lack of consensus regarding which type of antiplatelet agent should be used in patients with peripheral arterial disease (PAD) and little is known on the advantages and disadvantages of dual antiplatelet therapy. We conducted a systematic review and network meta-analysis of available randomized controlled trials (RCT) comparing different antiplatelet drugs (Aspirin, Ticlopidine, Clopidogrel, Ticagrelor, Cilostazol, Picotamide and Vorapaxar as monotherapies or in combination with aspirin) in PAD patients (PROSPERO public database; CRD42014010299).We collated evidence from previous relevant meta-analyses and searched online databases. Primary efficacy endpoints were: (1) the composite rate of major adverse cardiovascular events (MACE; including vascular deaths, non-fatal myocardial infarction and non-fatal stroke), and (2) the rate of major leg amputations. The primary safety endpoint was the rate of severe bleeding events. Bayesian models were employed for multiple treatment comparisons and risk-stratified hierarchies of comparative efficacy were produced to aid medical decision making. Number-Needed-to-Treat (NNT) and Number-Needed-to-Harm (NNH) are reported in case of significant results. We analyzed 49 RCTs comprising 34,518 patients with 88,358 person-years of follow-up with placebo as reference treatment. Aspirin, Cilostazol, Vorapaxar and Picotamide were ineffective in reducing MACE. A significant MACE reduction was noted with Ticagrelor plus aspirin (RR: 0.67; 95%CrI: 0.46-0.96, NNT = 66), Clopidogrel (RR: 0.72; 95%CrI: 0.58-0.91, NNT = 80), Ticlopidine (RR: 0.75; 95%CrI: 0.58-0.96, NNT = 87), and Clopidogrel plus aspirin (RR: 0.78; 95%CrI: 0.61-0.99, NNT = 98). Dual antiplatelet therapy with Clopidogrel plus aspirin significantly reduced major amputations following leg revascularization (RR: 0.68; 95%CrI: 0.46-0.99 compared to aspirin, NNT = 94). The risk of severe bleeding was significantly higher with Ticlopidine (RR: 5.03; 95%CrI: 1.23-39.6, NNH = 25), Vorapaxar (RR: 1.80; 95%CrI: 1.22-2.69, NNH = 130), and Clopidogrel plus aspirin (RR: 1.48; 95%CrI: 1.05-2.10, NNH = 215). Clopidogrel monotherapy showed the most favourable benefit-harm profile (79% cumulative rank probability best and 77% cumulative rank probability safest). In conclusion, Clopidogrel should be the indicated antiplatelet agent in PAD patients. Dual antiplatelet therapy with aspirin and Clopidogrel can reduce the rate of major leg amputations following revascularization, but carries a slightly higher risk of severe bleeding.
Conflict of interest statement
Figures






References
-
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–40. Epub 2013/08/07. 10.1016/S0140-6736(13)61249-0 . - DOI - PubMed
-
- NICE. Clinical Guideline 147: Lower Limb Peripheral Arterial Disease: Diagnosis and Management. National Clinical Guideline Centre; August 2012; Available: http://www.nice.org.uk.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

