Allergen-Specific Immunotherapy with Monomeric Allergoid in a Mouse Model of Atopic Dermatitis
- PMID: 26275152
- PMCID: PMC4537237
- DOI: 10.1371/journal.pone.0135070
Allergen-Specific Immunotherapy with Monomeric Allergoid in a Mouse Model of Atopic Dermatitis
Abstract
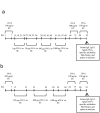
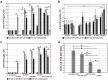
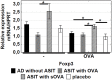
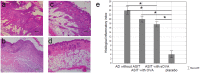
Atopic dermatitis (AD) is a widespread and difficult to treat allergic skin disease and is a tough challenge for healthcare. In this study, we investigated whether allergen-specific immunotherapy (ASIT) with a monomeric allergoid obtained by succinylation of ovalbumin (sOVA) is effective in a mouse model of atopic dermatitis. An experimental model of AD was reproduced by epicutaneous sensitization with ovalbumin (OVA). ASIT was performed with subcutaneous (SC) administration of increasing doses of OVA or sOVA. The levels of anti-OVA antibodies, as well as cytokines, were detected by ELISA. Skin samples from patch areas were taken for histologic examination. ASIT with either OVA or sOVA resulted in a reduction of both the anti-OVA IgE level and the IgG1/IgG2a ratio. Moreover, ASIT with sOVA increased the IFN-γ level in supernatants after splenocyte stimulation with OVA. Histologic analysis of skin samples from the sites of allergen application showed that ASIT improved the histologic picture by decreasing allergic inflammation in comparison with untreated mice. These data suggest that ASIT with a succinylated allergen represents promising approach for the treatment of AD.
Conflict of interest statement
Figures


References
-
- Bieber T, Novak N (2009). Pathogenesis of atopic dermatitis: new developments. Curr Allergy Asthma Rep 9:291–94. - PubMed
-
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. (2006). Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 38:441–46. - PubMed
-
- Kezic S, Novak N, Jakasa I, Jungersted JM, Simon M, Brandner JM, et al. (2014). Skin barrier in atopic dermatitis. Front Biosci (Landmark Ed) 19:542–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

