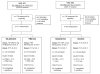
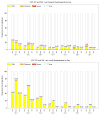
Phase I clinical evaluation of seasonal influenza hemagglutinin (HA) DNA vaccine prime followed by trivalent influenza inactivated vaccine (IIV3) boost
- PMID: 26275339
- PMCID: PMC4751067
- DOI: 10.1016/j.cct.2015.08.006
Phase I clinical evaluation of seasonal influenza hemagglutinin (HA) DNA vaccine prime followed by trivalent influenza inactivated vaccine (IIV3) boost
Abstract
Annual influenza vaccination reduces the risks of influenza when the vaccines are well matched to circulating strains, but development of an approach that induces broader and more durable immune responses would be beneficial. We conducted two companion Phase 1 studies, VRC 307 and VRC 309, over sequential seasons (2008-2009 and 2009-2010) in which only the influenza B strain component of the vaccines differed. Objectives were safety and immunogenicity of prime-boost vaccination schedules. A schedule of DNA vaccine encoding for seasonal influenza hemagglutinins (HA) prime followed by seasonal trivalent influenza inactivated vaccine (IIV3) boost (HA DNA-IIV3) was compared to placebo (PBS)-IIV3 or IIV3-IIV3. Cumulatively, 111 adults were randomized to HA DNA-IIV3 (n=66), PBS-IIV3 (n=25) or IIV3-IIV3 (n=20). Safety was assessed by clinical observations, laboratory parameters and 7-day solicited reactogenicity. The seasonal HA DNA prime-IIV3 boost regimen was evaluated as safe and well tolerated. There were no serious adverse events. The local and systemic reactogenicity for HA DNA, IIV and placebo were reported predominantly as none or mild within the first 5days post-vaccination. There was no significant difference in immunogenicity detected between the treatment groups as evaluated by hemagglutination inhibition (HAI) assay. The studies demonstrated the safety and immunogenicity of seasonal HA DNA-IIV3 regimen, but the 3-4week prime-boost interval was suboptimal for improving influenza-specific immune responses. This is consistent with observations in avian H5 DNA vaccine prime-boost studies in which a long interval, but not a short interval, was associated with improved immunogenicity.
Trial registration: NCT00858611 for VRC 307 and NCT00995982 for VRC 309.
Keywords: DNA vaccine; Immune response; Seasonal influenza.
Published by Elsevier Inc.
Figures
References
-
- Osterholm MT, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. - PubMed
-
- Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25(16):3066–3069. - PubMed
-
- CDC. Prevention, and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States 2012–13 in-fluenza season. MMWR Morb Mortal Wkly Rep. 2012;61(32):613–618. - PubMed
-
- Neuzil KM, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5–8-year-old children. J Infect Dis. 2006;194(8):1032–1039. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical