Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs
- PMID: 26275429
- PMCID: PMC4941182
- DOI: 10.1136/annrheumdis-2014-207178
Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs
Erratum in
-
Erratum: Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biologic diseasemodifying antirheumatic drugs.Ann Rheum Dis. 2017 Mar;76(3):611. doi: 10.1136/annrheumdis-2014-207178corr1. Ann Rheum Dis. 2017. PMID: 28213386 Free PMC article. No abstract available.
Abstract
Objectives: Biological disease-modifying antirheumatic drugs (bDMARDs) have shown diminished clinical response following an inadequate response (IR) to ≥1 previous bDMARD. Here, tofacitinib was compared with placebo in patients with an IR to conventional synthetic DMARDs (csDMARDs; bDMARD-naive) and in patients with an IR to bDMARDs (bDMARD-IR).
Methods: Data were taken from phase II and phase III studies of tofacitinib in patients with rheumatoid arthritis (RA). Patients received tofacitinib 5 or 10 mg twice daily, or placebo, as monotherapy or with background methotrexate or other csDMARDs. Efficacy endpoints and incidence rates of adverse events (AEs) of special interest were assessed.
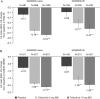
Results: 2812 bDMARD-naive and 705 bDMARD-IR patients were analysed. Baseline demographics and disease characteristics were generally similar between treatment groups within subpopulations. Across subpopulations, improvements in efficacy parameters at month 3 were generally significantly greater for both tofacitinib doses versus placebo. Clinical response was numerically greater with bDMARD-naive versus bDMARD-IR patients (overlapping 95% CIs). Rates of safety events of special interest were generally similar between tofacitinib doses and subpopulations; however, patients receiving glucocorticoids had more serious AEs, discontinuations due to AEs, serious infection events and herpes zoster. Numerically greater clinical responses and incidence rates of AEs of special interest were generally reported for tofacitinib 10 mg twice daily versus tofacitinib 5 mg twice daily (overlapping 95% CIs).
Conclusions: Tofacitinib demonstrated efficacy in both bDMARD-naive and bDMARD-IR patients with RA. Clinical response to tofacitinib was generally numerically greater in bDMARD-naive than bDMARD-IR patients. The safety profile appeared similar between subpopulations.
Trial registration numbers: (NCT00413660, NCT00550446, NCT00603512, NCT00687193, NCT00960440, NCT00847613, NCT00814307, NCT00856544, NCT00853385).
Keywords: Anti-TNF; DMARDs (biologic); DMARDs (synthetic); Rheumatoid Arthritis; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

References
-
- Bresnihan B. Rheumatoid arthritis: principles of early treatment. J Rheumatol Suppl 2002;66:9–12. http://www.ncbi.nlm.nih.gov/pubmed/12435163 - PubMed
-
- Emery P. Evidence supporting the benefit of early intervention in rheumatoid arthritis. J Rheumatol Suppl 2002;66:3–8. http://www.ncbi.nlm.nih.gov/pubmed/12435162 - PubMed
-
- Furst DE, Pangan AL, Harrold LR, et al. . Greater likelihood of remission in rheumatoid arthritis patients treated earlier in the disease course: results from the Consortium of Rheumatology Researchers of North America registry. Arthritis Care Res (Hoboken) 2011;63:856–64. 10.1002/acr.20452 - DOI - PMC - PubMed
-
- Heidari B. Rheumatoid Arthritis: early diagnosis and treatment outcomes. Caspian J Intern Med 2011;2:161–70. http://www.ncbi.nlm.nih.gov/pubmed/24024009 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

