Flagellar membranes are rich in raft-forming phospholipids
- PMID: 26276100
- PMCID: PMC4582118
- DOI: 10.1242/bio.011957
Flagellar membranes are rich in raft-forming phospholipids
Abstract
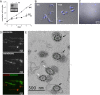
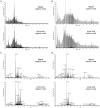
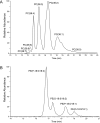
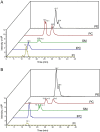
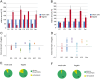
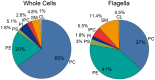
The observation that the membranes of flagella are enriched in sterols and sphingolipids has led to the hypothesis that flagella might be enriched in raft-forming lipids. However, a detailed lipidomic analysis of flagellar membranes is not available. Novel protocols to detach and isolate intact flagella from Trypanosoma brucei procyclic forms in combination with reverse-phase liquid chromatography high-resolution tandem mass spectrometry allowed us to determine the phospholipid composition of flagellar membranes relative to whole cells. Our analyses revealed that phosphatidylethanolamine, phosphatidylserine, ceramide and the sphingolipids inositol phosphorylceramide and sphingomyelin are enriched in flagella relative to whole cells. In contrast, phosphatidylcholine and phosphatidylinositol are strongly depleted in flagella. Within individual glycerophospholipid classes, we observed a preference for ether-type over diacyl-type molecular species in membranes of flagella. Our study provides direct evidence for a preferential presence of raft-forming phospholipids in flagellar membranes of T. brucei.
Keywords: Flagella; Lipid rafts; Mass spectrometry; Membrane lipids; Sphingolipids; Trypanosoma brucei.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

