Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges
- PMID: 26276218
- PMCID: PMC4627459
- DOI: 10.1208/s12248-015-9814-9
Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges
Abstract
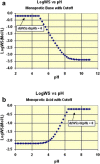
Various drug delivery approaches can be used to maximize therapeutic efficacy and minimize side effects, by impacting absorption, distribution, metabolism, and elimination (ADME) of a drug compound. For those drugs with poor water solubility or low permeability, techniques such as amorphous solid dispersion, liposomes, and complexations have been used to improve their oral bioavailability. Modified release (MR) formulations have been widely used to improve patient compliance, as well as to reduce side effects, especially for those drugs with short half-lives or narrow therapeutic windows. More than ten drugs using sterile long-acting release (LAR) formulations with clear clinical benefit have been successfully marketed. Furthermore, drug delivery systems have been used in delaying drug clearance processes. Additionally, modifying the in vivo drug distribution using targeted delivery systems has significantly improved oncology treatments. All the drug delivery approaches have their advantages and limitations. For both brand and generic drugs, the achievement of consistent quality and therapeutic performance using drug delivery systems can also pose serious challenges in developing a drug for the market, which requires close collaboration among industry, academia, and regulatory agencies. With the advent of personalized medicines, there will be great opportunities and challenges in utilizing drug delivery systems to provide better products and services for patients.
Keywords: absorption, distribution, metabolism, and elimination (ADME); adverse effects; bioequivalence; clinical pharmacology; drug delivery; formulation design; local delivery; long-acting release; modified release; personalized medicine; pharmacokinetic profiles; prodrug; quality; regulatory; targeted delivery; therapeutic performance.
Figures
References
-
- Drug delivery. Available from: http://en.wikipedia.org/wiki/Drug_delivery.
-
- Guidance for industry and review staff: target product profile—a strategic development process tool. 2007; Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformati....
-
- Advanced delivery devices - wearable bolus injectors - a new class of patient-friendly drug delivery systems 2014; Available from: http://www.drug-dev.com/Main/Back-Issues/ADVANCED-DELIVERY-DEVICES-Weara....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous