Antisense-mediated affinity purification of dengue virus ribonucleoprotein complexes from infected cells
- PMID: 26276314
- PMCID: PMC4684824
- DOI: 10.1016/j.ymeth.2015.08.008
Antisense-mediated affinity purification of dengue virus ribonucleoprotein complexes from infected cells
Abstract
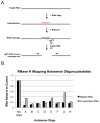
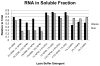
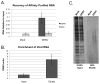
The identification of RNA-binding proteins that physically associate with viral RNA molecules during infection can provide insight into the molecular mechanisms of RNA virus replication. Until recently, such RNA-protein interactions have been identified predominantly with the use of in vitro assays that may not accurately reflect associations that occur in the context of a living cell. Here we describe a method for the specific affinity purification of dengue virus RNA and associated proteins using in vivo cross-linking followed by antisense-mediated affinity purification. RNA-binding proteins that specifically co-purify with viral RNA using this method can be identified en masse by mass spectrometry. This strategy can potentially be adapted to the purification of any viral RNA species of interest.
Keywords: Dengue virus; RNA affinity chromatography; Ribonucleoprotein complex.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
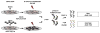
References
-
- Deo S, et al. Characterization of the termini of the West Nile virus genome and their interactions with the small isoform of the 2′ 5′-oligoadenylate synthetase family. J Struct Biol. 2015;190(2):236–49. - PubMed
-
- Paranjape SM, Harris E. Y box-binding protein-1 binds to the dengue virus 3′-untranslated region and mediates antiviral effects. J Biol Chem. 2007;282(42):30497–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

