A Conserved Bicycle Model for Circadian Clock Control of Membrane Excitability
- PMID: 26276633
- PMCID: PMC4537776
- DOI: 10.1016/j.cell.2015.07.036
A Conserved Bicycle Model for Circadian Clock Control of Membrane Excitability
Abstract
Circadian clocks regulate membrane excitability in master pacemaker neurons to control daily rhythms of sleep and wake. Here, we find that two distinctly timed electrical drives collaborate to impose rhythmicity on Drosophila clock neurons. In the morning, a voltage-independent sodium conductance via the NA/NALCN ion channel depolarizes these neurons. This current is driven by the rhythmic expression of NCA localization factor-1, linking the molecular clock to ion channel function. In the evening, basal potassium currents peak to silence clock neurons. Remarkably, daily antiphase cycles of sodium and potassium currents also drive mouse clock neuron rhythms. Thus, we reveal an evolutionarily ancient strategy for the neural mechanisms that govern daily sleep and wake.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
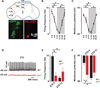

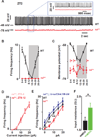
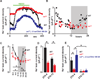



References
-
- Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, Stewart AF, Schutz G. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis. 2001;31:37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS074257/NS/NINDS NIH HHS/United States
- R01 NS055293/NS/NINDS NIH HHS/United States
- R01 NS052903/NS/NINDS NIH HHS/United States
- NS055293/NS/NINDS NIH HHS/United States
- T32GM07281/GM/NIGMS NIH HHS/United States
- T32 GM007281/GM/NIGMS NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01MH092273/MH/NIMH NIH HHS/United States
- R01 MH092273/MH/NIMH NIH HHS/United States
- R00 GM080107/GM/NIGMS NIH HHS/United States
- R01 NS074257/NS/NINDS NIH HHS/United States
- NS054850/NS/NINDS NIH HHS/United States
- R01NS052903/NS/NINDS NIH HHS/United States
- P30 NS054850/NS/NINDS NIH HHS/United States
- R00GM080107/GM/NIGMS NIH HHS/United States
- CA060553/CA/NCI NIH HHS/United States
- R01 GM084947/GM/NIGMS NIH HHS/United States
- R01-GM084947/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

