Tau deposition drives neuropathological, inflammatory and behavioral abnormalities independently of neuronal loss in a novel mouse model
- PMID: 26276810
- PMCID: PMC4599677
- DOI: 10.1093/hmg/ddv336
Tau deposition drives neuropathological, inflammatory and behavioral abnormalities independently of neuronal loss in a novel mouse model
Abstract
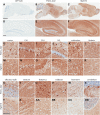
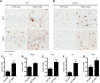
Aberrant tau protein accumulation drives neurofibrillary tangle (NFT) formation in several neurodegenerative diseases. Currently, efforts to elucidate pathogenic mechanisms and assess the efficacy of therapeutic targets are limited by constraints of existing models of tauopathy. In order to generate a more versatile mouse model of tauopathy, somatic brain transgenesis was utilized to deliver adeno-associated virus serotype 1 (AAV1) encoding human mutant P301L-tau compared with GFP control. At 6 months of age, we observed widespread human tau expression with concomitant accumulation of hyperphosphorylated and abnormally folded proteinase K resistant tau. However, no overt neuronal loss was observed, though significant abnormalities were noted in the postsynaptic scaffolding protein PSD95. Neurofibrillary pathology was also detected with Gallyas silver stain and Thioflavin-S, and electron microscopy revealed the deposition of closely packed filaments. In addition to classic markers of tauopathy, significant neuroinflammation and extensive gliosis were detected in AAV1-Tau(P301L) mice. This model also recapitulates the behavioral phenotype characteristic of mouse models of tauopathy, including abnormalities in exploration, anxiety, and learning and memory. These findings indicate that biochemical and neuropathological hallmarks of tauopathies are accurately conserved and are independent of cell death in this novel AAV-based model of tauopathy, which offers exceptional versatility and speed in comparison with existing transgenic models. Therefore, we anticipate this approach will facilitate the identification and validation of genetic modifiers of disease, as well as accelerate preclinical assessment of potential therapeutic targets.
© The Author 2015. Published by Oxford University Press.
Figures







References
-
- Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A. et al. (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature, 393, 702–705. - PubMed
-
- Poorkaj P., Bird T.D., Wijsman E., Nemens E., Garruto R.M., Anderson L., Andreadis A., Wiederholt W.C., Raskind M., Schellenberg G.D. (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol., 43, 815–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

