Rapid multi-orientation quantitative susceptibility mapping
- PMID: 26277773
- PMCID: PMC4691433
- DOI: 10.1016/j.neuroimage.2015.08.015
Rapid multi-orientation quantitative susceptibility mapping
Abstract
Three-dimensional gradient echo (GRE) is the main workhorse sequence used for susceptibility weighted imaging (SWI), quantitative susceptibility mapping (QSM), and susceptibility tensor imaging (STI). Achieving optimal phase signal-to-noise ratio requires late echo times, thus necessitating a long repetition time (TR). Combined with the large encoding burden of whole-brain coverage with high resolution, this leads to increased scan time. Further, the dipole kernel relating the tissue phase to the underlying susceptibility distribution undersamples the frequency content of the susceptibility map. Scans at multiple head orientations along with calculation of susceptibility through multi-orientation sampling (COSMOS) are one way to effectively mitigate this issue. Additionally, STI requires a minimum of 6 head orientations to solve for the independent tensor elements in each voxel. The requirements of high-resolution imaging with long TR at multiple orientations substantially lengthen the acquisition of COSMOS and STI. The goal of this work is to dramatically speed up susceptibility mapping at multiple head orientations. We demonstrate highly efficient acquisition using 3D-GRE with Wave-CAIPI and dramatically reduce the acquisition time of these protocols. Using R=15-fold acceleration with Wave-CAIPI permits acquisition per head orientation in 90s at 1.1mm isotropic resolution, and 5:35min at 0.5mm isotropic resolution. Since Wave-CAIPI fully harnesses the 3D spatial encoding capability of receive arrays, the maximum g-factor noise amplification remains below 1.30 at 3T and 1.12 at 7T. This allows a 30-min exam for STI with 12 orientations, thus paving the way to its clinical application.
Keywords: Parallel imaging; Phase imaging; Quantitative susceptibility mapping; Susceptibility tensor imaging; Wave-CAIPI.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
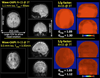









Similar articles
-
Simultaneous Time Interleaved MultiSlice (STIMS) for Rapid Susceptibility Weighted acquisition.Neuroimage. 2017 Jul 15;155:577-586. doi: 10.1016/j.neuroimage.2017.04.036. Epub 2017 Apr 20. Neuroimage. 2017. PMID: 28435102 Free PMC article.
-
Single-step quantitative susceptibility mapping with variational penalties.NMR Biomed. 2017 Apr;30(4):10.1002/nbm.3570. doi: 10.1002/nbm.3570. Epub 2016 Jun 22. NMR Biomed. 2017. PMID: 27332141 Free PMC article.
-
Wave-CAIPI for highly accelerated 3D imaging.Magn Reson Med. 2015 Jun;73(6):2152-62. doi: 10.1002/mrm.25347. Epub 2014 Jul 1. Magn Reson Med. 2015. PMID: 24986223 Free PMC article.
-
Susceptibility tensor imaging (STI) of the brain.NMR Biomed. 2017 Apr;30(4):10.1002/nbm.3540. doi: 10.1002/nbm.3540. Epub 2016 Apr 27. NMR Biomed. 2017. PMID: 27120169 Free PMC article. Review.
-
Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain.J Magn Reson Imaging. 2015 Jul;42(1):23-41. doi: 10.1002/jmri.24768. Epub 2014 Oct 1. J Magn Reson Imaging. 2015. PMID: 25270052 Free PMC article. Review.
Cited by
-
DeepSTI: Towards tensor reconstruction using fewer orientations in susceptibility tensor imaging.Med Image Anal. 2023 Jul;87:102829. doi: 10.1016/j.media.2023.102829. Epub 2023 Apr 26. Med Image Anal. 2023. PMID: 37146440 Free PMC article.
-
Validation of Highly Accelerated Wave-CAIPI SWI Compared with Conventional SWI and T2*-Weighted Gradient Recalled-Echo for Routine Clinical Brain MRI at 3T.AJNR Am J Neuroradiol. 2019 Dec;40(12):2073-2080. doi: 10.3174/ajnr.A6295. Epub 2019 Nov 14. AJNR Am J Neuroradiol. 2019. PMID: 31727749 Free PMC article.
-
Simultaneous Time Interleaved MultiSlice (STIMS) for Rapid Susceptibility Weighted acquisition.Neuroimage. 2017 Jul 15;155:577-586. doi: 10.1016/j.neuroimage.2017.04.036. Epub 2017 Apr 20. Neuroimage. 2017. PMID: 28435102 Free PMC article.
-
Single-step quantitative susceptibility mapping with variational penalties.NMR Biomed. 2017 Apr;30(4):10.1002/nbm.3570. doi: 10.1002/nbm.3570. Epub 2016 Jun 22. NMR Biomed. 2017. PMID: 27332141 Free PMC article.
-
Asymmetric susceptibility tensor imaging.Magn Reson Med. 2021 Oct;86(4):2266-2275. doi: 10.1002/mrm.28823. Epub 2021 May 20. Magn Reson Med. 2021. PMID: 34014008 Free PMC article.
References
-
- Breuer F, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA) Magnetic Resonance in Medicine. 2006;55.3:549–556. - PubMed
-
- Conolly S, Nishimura D, Mackovski A, Glover G. Variable-rate selective excitation. Journal of Magnetic Resonance. 1988;78(3):440–458.
Publication types
MeSH terms
Grants and funding
- R01 MH096979/MH/NIMH NIH HHS/United States
- P41 EB015897/EB/NIBIB NIH HHS/United States
- U01 MH093765/MH/NIMH NIH HHS/United States
- 1U01MH093765/MH/NIMH NIH HHS/United States
- R01 NS079653/NS/NINDS NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- R00EB012107/EB/NIBIB NIH HHS/United States
- R01 EB017337/EB/NIBIB NIH HHS/United States
- R00 EB012107/EB/NIBIB NIH HHS/United States
- R21 HL122759/HL/NHLBI NIH HHS/United States
- R01NS079653/NS/NINDS NIH HHS/United States
- P41RR14075/RR/NCRR NIH HHS/United States
- R01MH096979/MH/NIMH NIH HHS/United States
- J 3480/FWF_/Austrian Science Fund FWF/Austria
- R01EB017337/EB/NIBIB NIH HHS/United States
- R21-HL122759/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

