Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site
- PMID: 26279189
- PMCID: PMC4757492
- DOI: 10.1016/j.cell.2015.07.043
Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site
Abstract
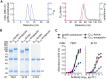
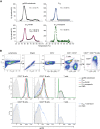
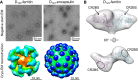
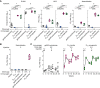
Epstein-Barr virus (EBV) represents a major global health problem. Though it is associated with infectious mononucleosis and ∼200,000 cancers annually worldwide, a vaccine is not available. The major target of immunity is EBV glycoprotein 350/220 (gp350) that mediates attachment to B cells through complement receptor 2 (CR2/CD21). Here, we created self-assembling nanoparticles that displayed different domains of gp350 in a symmetric array. By focusing presentation of the CR2-binding domain on nanoparticles, potent neutralizing antibodies were elicited in mice and non-human primates. The structurally designed nanoparticle vaccine increased neutralization 10- to 100-fold compared to soluble gp350 by targeting a functionally conserved site of vulnerability, improving vaccine-induced protection in a mouse model. This rational approach to EBV vaccine design elicited potent neutralizing antibody responses by arrayed presentation of a conserved viral entry domain, a strategy that can be applied to other viruses.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




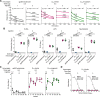
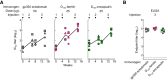


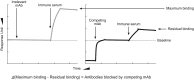
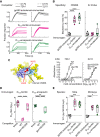
References
-
- Bachmann M.F., Zinkernagel R.M. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 1997;15:235–270. - PubMed
-
- Bachmann M.F., Kalinke U., Althage A., Freer G., Burkhart C., Roost H., Aguet M., Hengartner H., Zinkernagel R.M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. - PubMed
-
- Blasco R., Moss B. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene. 1995;158:157–162. - PubMed
-
- Chen Z., Earl P., Americo J., Damon I., Smith S.K., Yu F., Sebrell A., Emerson S., Cohen G., Eisenberg R.J. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. J. Virol. 2007;81:8989–8995. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

