Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves
- PMID: 26279190
- PMCID: PMC4553238
- DOI: 10.1016/j.cell.2015.07.021
Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves
Abstract
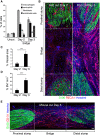
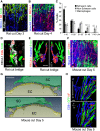
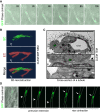
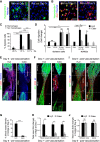
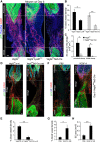
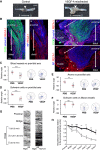
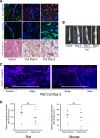
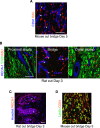
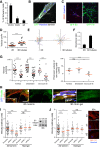
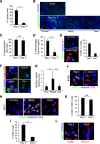
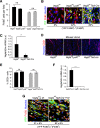
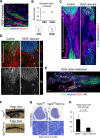
The peripheral nervous system has remarkable regenerative capacities in that it can repair a fully cut nerve. This requires Schwann cells to migrate collectively to guide regrowing axons across a 'bridge' of new tissue, which forms to reconnect a severed nerve. Here we show that blood vessels direct the migrating cords of Schwann cells. This multicellular process is initiated by hypoxia, selectively sensed by macrophages within the bridge, which via VEGF-A secretion induce a polarized vasculature that relieves the hypoxia. Schwann cells then use the blood vessels as "tracks" to cross the bridge taking regrowing axons with them. Importantly, disrupting the organization of the newly formed blood vessels in vivo, either by inhibiting the angiogenic signal or by re-orienting them, compromises Schwann cell directionality resulting in defective nerve repair. This study provides important insights into how the choreography of multiple cell-types is required for the regeneration of an adult tissue.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Avellino A.M., Hart D., Dailey A.T., MacKinnon M., Ellegala D., Kliot M. Differential macrophage responses in the peripheral and central nervous system during wallerian degeneration of axons. Exp. Neurol. 1995;136:183–198. - PubMed
-
- Bussmann J., Bos F.L., Urasaki A., Kawakami K., Duckers H.J., Schulte-Merker S. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development. 2010;137:2653–2657. - PubMed

