In vivo micro-scale tomography of ciliary behavior in the mammalian oviduct
- PMID: 26279472
- PMCID: PMC4538602
- DOI: 10.1038/srep13216
In vivo micro-scale tomography of ciliary behavior in the mammalian oviduct
Abstract
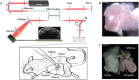
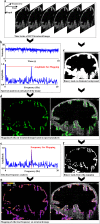
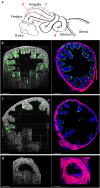
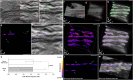
Motile cilia in the mammalian oviduct play a key role in reproduction, such as transporting fertilized oocytes to the uterus for implantation. Due to their small size (~5-10 μm in length and ~300 nm in diameter), live visualization of cilia and their activity in the lumen of the oviduct through tissue layers represents a major challenge not yet overcome. Here, we report a functional low-coherence optical imaging technique that allows in vivo depth-resolved mapping of the cilia location and cilia beat frequency (CBF) in the intact mouse oviduct with micro-scale spatial resolution. We validate our approach with widely-used microscopic imaging methods, present the first in vivo mapping of the oviduct CBF in its native context, and demonstrate the ability of this approach to differentiate CBF in different locations of the oviduct at different post-conception stages. This technique opens a range of opportunities for live studies in reproductive medicine as well as other areas focused on cilia activity and related ciliopathies.
Figures

References
-
- Salathe M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 69, 401–422 (2007). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

