S-Ribosylhomocysteine analogs containing a [4-thio]ribose ring
- PMID: 26279525
- PMCID: PMC4604056
- DOI: 10.1016/j.carres.2015.07.005
S-Ribosylhomocysteine analogs containing a [4-thio]ribose ring
Abstract
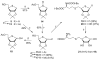
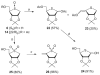
The [4-thio]-S-ribosylhomocysteine (SRH) analogs containing substitution of a sulfur atom for the endocyclic oxygen were synthesized by coupling of the 4-thioribose substrates with a thiolate generated from the protected homocysteine. Coupling of the protected 1-deoxy-5-O-mesyl-S-oxo-4-thio-D-ribofuranose with homocysteinate salt gave the C4 epimers of [4-thio]-SRH at the sulfoxide oxidation level lacking a hydroxyl group at anomeric carbon. Treatment of these sulfoxides with BF3⋅Et2O/NaI affected simultaneous reduction to sulfide and global deprotection affording 1-deoxy-4-thio-SRH analog. Treatment of the protected 1-deoxy-S-oxo-4-thio-D-ribofuranose sulfoxide with DAST/SbCl3 resulted in the fluoro-Pummerer rearrangement to give 4-thio-β-D-ribofuranosyl fluoride. Mesylation of the latter at 5-hydroxyl position followed by coupling with homocysteinate salt and subsequent global deprotection with trifluoroacetic acid afforded [4-thio]-SRH thiohemiacetal.
Keywords: Homocysteine; LuxS; S-Ribosylhomocysteine; Thioacetals; Thiosugars.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

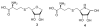

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

